 | |
| Names | |
|---|---|
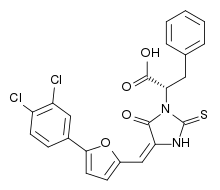
| IUPAC name
(S,E)-2-(4-((5-(3,4-dichlorophenyl)furan-2-yl)methylene)-5-oxo-2-thioxoimidazolidin-1-yl)-3-phenylpropanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C23H16Cl2N2O4S | |
| Molar mass | 487.35 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
 | |
| Names | |
|---|---|
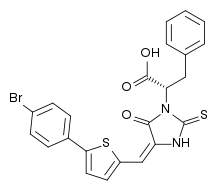
| IUPAC name
(S,E)-2-(4-((5-(3-bromophenyl)thiophene-2-yl)methylene)-5-oxo-2-thioxoimidazolidin-1-yl)-3-phenylpropanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C23H17BrN2O3S2 | |
| Molar mass | 513.42 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Epimerox is an experimental broad-spectrum antibiotic compound being developed by scientists at the Rockefeller University and Astex Pharmaceuticals. It is a small molecule inhibitor compound that blocks the activity of the enzyme UDP-N-acetylglucosamine 2-epimerase, an epimerase enzyme[1][2] that is called 2-epimerase for short.[3]
Mechanism of action
2-Epimerase converts UDP-N-acetyl-D-glucosamine to UDP-N-acetyl-D-mannosamine. Bacterial 2-epimerase differs from its human counterpart in that the bacterial molecule has an allosteric site.[4] For enzymatic activity, the allosteric site needs to be occupied by the substrate UDP-N-acetylglucosamine; this is the first enzyme described in which the substrate is present in both the catalytic and allosteric sites. Because epimerox targets the allosteric site of the bacterial 2-epimerase, low human toxicity is expected since the human 2-epimerase does not have this site.[4]
Although epimerox was originally developed specifically to target Bacillus anthracis, the bacterium that causes anthrax, it was found that it is also effective against methicillin-resistant Staphylococcus aureus (MRSA),[3] and many other Gram-positive bacteria.[2][5]
No bacteria could be identified that were resistant to the compound (resistance frequency of <10−11). Thus, epimerox is an antibiotic with low resistance potential. Therefore, 2-epimerase is a new antibiotic target to which resistance is a rare event. 2-Epimerase was discovered as a target because it was one of the enzymes in the biosynthetic pathway for synthesizing an essential neutral polysaccharide in the cell wall of B. anthracis (Glu:Gal-NAc:Man-NAc at a 3:2:1 ratio).[6] This polysaccharide is only found in B. anthracis and is the receptor for a bacteriophage lysin enzyme called PlyG, which is produced by the γ-bacteriophage to release its progeny from infected B. anthracis. For bacteriophage to survive they need to replicate inside a bacterial cell and release their progeny phage when they are assembled using their lysin. Since lysins from bacteriophage that infect Gram-positive bacteria must bind to a cell wall receptor to function, the enzymes have evolved over a billion years to identify substrate receptors in the bacterial cell wall that the bacteria cannot change easily. These substrates are either part of the peptidoglycan or sugars linked to it. Deletion of both 2-epimerase genes in B. anthracis is lethal to the bacterium.[3]
Chemistry
Epimerox is the name of two chemically closely related compounds that were found to block 2-epimerase and act as antibiotics by the described mechanism. Both are thiohydantoin derivatives of the amino acid phenylalanine. They differ in the residue on the thiohydantoin's carbon atom, which is (E)-5-(3,4-dichlorophenyl)furan-2-ylmethylene in one compound and (E)-5-(3-bromophenyl)thiophene-2-ylmethylene in the other.
Synthesis
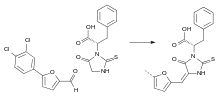
The substances are prepared via a Knoevenagel condensation of a thiohydantoin–phenylalanine imine with different aldehydes, which yields a mixture of E and Z isomers. The active compounds are the E forms.[3]
References
- ↑ "A New Era: The Fight Against Antibiotic-Resistant Pathogenic Bacteria". New York Academy of Sciences. December 21, 2011.
- 1 2 Michael Manning (April 14, 2013). "New Anthrax-Killing Antibiotic Also Eliminates MRSA".
- 1 2 3 4 Schuch, R.; Pelzek, A. J.; Raz, A.; Euler, C. W.; Ryan, P. A.; Winer, B. Y.; Farnsworth, A.; Bhaskaran, S. S.; Stebbins, C. E.; Xu, Y.; Clifford, A.; Bearss, D. J.; Vankayalapati, H.; Goldberg, A. R.; Fischetti, V. A. (2013). Aziz, Ramy K (ed.). "Use of a Bacteriophage Lysin to Identify a Novel Target for Antimicrobial Development". PLOS ONE. 8 (4): e60754. Bibcode:2013PLoSO...860754S. doi:10.1371/journal.pone.0060754. PMC 3622686. PMID 23593301.
- 1 2 Velloso, L.M.; Bhaskaran SS; Schuch R; Fischetti VA; Stebbins CE. (2008). "A structural basis for the allosteric regulation of non-hydrolysing UDP-GlcNAc 2-epimerases". EMBO Rep. 9 (2): 199–205. doi:10.1038/sj.embor.7401154. PMC 2246411. PMID 18188181.
- ↑ "MRSA-killing Antibiotic Developed". Bioscience Technology. April 12, 2013.
- ↑ Choudhury, B; Leoff C; Saile E; Wilkins P; Quinn CP; et al. (22 Sep 2006). "The structure of the major cell wall polysaccharide of Bacillus anthracis is species-specific". J. Biol. Chem. 281 (38): 27932–27941. doi:10.1074/jbc.M605768200. PMID 16870610.