 | |
| Clinical data | |
|---|---|
| Other names | WIN-32729 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
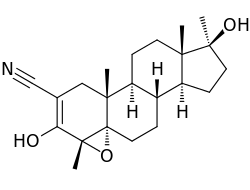
| Formula | C22H31NO3 |
| Molar mass | 357.494 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Epostane (INN, USAN, BAN) (developmental code name WIN-32729) is an inhibitor of 3β-hydroxysteroid dehydrogenase (3β-HSD) that was developed as a contraceptive, abortifacient, and oxytocic drug but was never marketed.[1][2][3] By inhibiting 3β-HSD, epostane blocks the biosynthesis of progesterone from pregnenolone (and also the conversion of dehydroepiandrosterone to androstenedione), thereby functioning as an antiprogestogen and terminating pregnancy.[1] The drug was trialed and in a study was found to be slightly more effective at inducing abortion relative to mifepristone.[4]
See also
References
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 492–. ISBN 978-1-4757-2085-3.
- ↑ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 113–. ISBN 978-94-011-4439-1.
- ↑ Milne GW (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 23–. ISBN 978-1-351-78989-9.
- ↑ Lachelin GC (11 September 2013). Introduction to Clinical Reproductive Endocrinology. Elsevier Science. pp. 198–. ISBN 978-1-4831-9380-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.