 | |
| Names | |
|---|---|
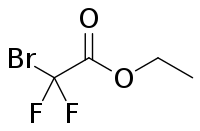
| IUPAC name
Ethyl 2-bromo-2,2-difluoroacetate | |
| Systematic IUPAC name
Ethyl 2,2-dibromo-2-fluoro-acetate | |
| Other names
Difluorobromoacetic acid ethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.517 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H5BrF2O2 | |
| Molar mass | 202.983 g·mol−1 |
| Appearance | clear colorless to slightly yellow liquid |
| Density | 1.583 g/mL |
| Boiling point | 82 °C (180 °F; 355 K) pressure is at 33 torr |
| Vapor pressure | 1.36 mmHg at 25°C |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ethyl bromodifluoroacetate is an ester that can be used to introduce the CF2 group when synthesising chemical compounds. It is a clear to yellow liquid. It is an ester of bromodifluoroacetic acid and ethyl alcohol.
Formation
Ethyl fluorosulfonoxydifluoroacetate can react with sodium bromide (NaBr) to produce ethyl bromodifluoroacetate. And this reaction could happen in the solvent sulfolane. The reactions takes 12 hours at 100 °C with a yield of 31%.
Reactions
Ethyl bromodifluoroacetate, and other similar compounds containing a CF2 units can be generated using the Reformatsky reagent with aldehydes and ketones. This yields 2,2-difluoro-3-hydroxy esters. Also ethyl bromdifluoroacetate is considered to be a good compound in generation of compounds and for testing with other organic compounds like lactones, imines and other amino acids.[2]
References
- ↑ datasheet, Santa Cruz Biotechnology, 2012
- ↑ Sato, Kazuyuki; Tamura, Misato; Tamoto, Kei; Omote, Masaaki; Ando, Akira; Kumadaki, Itsumaro (2000). "Michael-type Reaction of Ethyl Bromodifluoroacetate with α,β-Unsaturated Carbonyl Compounds in the Presence of Copper Powder" (PDF). Chemical and Pharmaceutical Bulletin. 48 (7): 1023–1025. doi:10.1248/cpb.48.1023. PMID 10923834.