 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.011.935 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5BrMg | |
| Molar mass | 133.271 g·mol−1 |
| Hazards | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
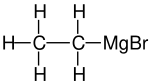
Ethylmagnesium bromide is a Grignard reagent with formula C2H5MgBr. It is widely used in the laboratory synthesis of organic compounds.
Reactions
Apart from acting as the synthetic equivalent of an ethyl anion synthon for nucleophilic addition, ethylmagnesium bromide may be used as a strong base to deprotonate various substrates such as alkynes:[1][2][3]
- RC≡CH + EtMgBr → RC≡CMgBr + EtH
In this application, ethylmagnesium bromide has been supplanted by the wide availability of organolithium reagents.
Preparation
Ethylmagnesium bromide is commercially available, usually as a solution in diethyl ether or tetrahydrofuran. It may be prepared in the normal manner of Grignard reagents — by reacting bromoethane with magnesium in diethyl ether:[4]
- EtBr + Mg → EtMgBr
References
- ↑ Taniguchi, H.; Mathai, I. M.; Miller, S. I. (1970). "1-Phenyl-1,4-Pentadiyne and 1-Phenyl-1,3-Pentadiyne". Organic Syntheses. 50: 97.; Collective Volume, vol. 6, p. 925
- ↑ Quillinan, A. J.; Scheinmann, F. (1978). "3-Alkyl-1-alkynes Synthesis: 3-Ethyle-1-hexyne". Organic Syntheses. 58: 1.; Collective Volume, vol. 6, p. 595
- ↑ Newman, M. S.; Stalick, W. M. (1977). "1-Ethoxy-1-butyne". Organic Syntheses. 57: 65.; Collective Volume, vol. 6, p. 564
- ↑ Moyer, W. W.; Marvel, C. S. (1931). "Triethyl Carbinol". Organic Syntheses. 11: 98.; Collective Volume, vol. 2, p. 602
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.