 | |
 | |
| Names | |
|---|---|
| Other names
Eu(fod)3; Sievers' Reagent; Tris(6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octanedionato)europium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.037.817 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H30EuF21O6 | |
| Molar mass | 1037.49 g/mol |
| Appearance | Yellow powder |
| Melting point | 203 to 207 °C (397 to 405 °F; 476 to 480 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
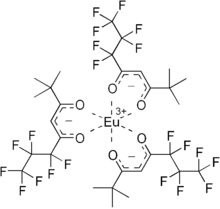
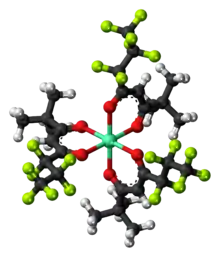
EuFOD is the chemical compound with the formula Eu(OCC(CH3)3CHCOC3F7)3, also called Eu(fod)3. This coordination compound is used primarily as a shift reagent in NMR spectroscopy. It is the premier member of the lanthanide shift reagents and was popular in the 1970s and 1980s.
Structure and reactivity
Eu(fod)3 consists of three bidentate ligands bound to a Eu(III) center. The metal atom has an electron configuration of f6. The six electrons are unpaired—each in a different singly-occupied f-orbital—which makes the molecule highly paramagnetic. In contrast, Gd(fod)3 with a symmetrical f7 configuration, does not give rise to pseudocontact shifts. The complex is a Lewis acid, being capable of expanding its coordination number of six to eight. The complex displays a particular affinity for "hard" Lewis bases, such as the oxygen atom in ethers and the nitrogen of amines. It is soluble in nonpolar solvents, even more so than related complexes of acetylacetone and hexafluoroacetylacetone.
The fod ligand is the anion of the commercially available 6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octanedione. It is a bidentate acetylacetonate ligand prepared from heptafluorobutyric acid (PFBA). It chelates with lanthanides to form Ln(fod)3 for La = Nd, Sm, Eu, Tb, and Lu.[1]
Uses
NMR shift reagent
In its original application, Eu(fod)3 was used in NMR spectroscopy to gain additional chemical shift dispersion. As is typical in paramagnetic NMR spectroscopy, the paramagnetic compound induces an additional chemical shift in the protons near those Lewis basic sites that bind to Eu(fod)3. Only small amounts of shift reagents are used, because otherwise the paramagnetism of the reagent shortens the spin-lattice relaxation times of the nuclei, which causes uncertainty broadening and loss of resolution. The availability of higher magnetic field spectrometers has lowered the demand for NMR shift reagents.
The original shift reagent was Eu(DPM)3,[2][3] also called Eu(thd)3.[4] Its structure is similar to EuFOD, but with tert-butyl groups in place of heptafluoropropyl substituents. That is, DPM− is the conjugate base derived from dipivaloylmethane, also known as 2,2,6,6-tetramethylheptane-3,5-dione. The ligand fod− is more lipophilic and by virtue of the perfluoralkyl substituent, its complexes are more Lewis acidic than those derived from DPM−.[4]
Lewis acid
Eu(fod)3 serves as a Lewis acid catalyst in organic synthesis including stereoselective Diels-Alder and aldol addition reactions. For example, Eu(fod)3 catalyzes the cyclocondensations of substituted dienes with aromatic and aliphatic aldehydes to yield dihydropyrans, with high selectivity for the endo product.[5]
See also
- TRISPHAT, a chiral shift reagent for cations
References
- ↑ Irfanullah, Mir; Iftikhar, K. (2009). "New dinuclear lanthanide(III) complexes based on 6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octanedione and 2,2′-bipyrimidine". Inorg. Chem. Comm. 12 (4): 296–299. doi:10.1016/j.inoche.2009.01.007. ISSN 1387-7003.
- ↑ Hinckley, Conrad C. (1969). "Paramagnetic shifts in solutions of cholesterol and the dipyridine adduct of trisdipivalomethanatoeuropium(III). A shift reagent". J. Am. Chem. Soc. 91 (18): 5160–5162. doi:10.1021/ja01046a038. ISSN 0002-7863. PMID 5798101.
- ↑ Sanders, Jeremy K. M.; Williams, Dudley H. (1972). "Shift Reagents in NMR Spectroscopy". Nature. 240 (5381): 385–390. Bibcode:1972Natur.240..385S. doi:10.1038/240385a0. ISSN 0028-0836. S2CID 4275735.
- 1 2 Sievers, Robert E.; Rondeau, Roger E. (1971). "New superior paramagnetic shift reagents for nuclear magnetic resonance spectral clarification". J. Am. Chem. Soc. 93 (6): 1522–1524. doi:10.1021/ja00735a049. ISSN 0002-7863.
- ↑ Wenzel, T.J.; Ciak, J.M.; "Europium, tris(6,6,7,7,8,8,8-heptafluoro-2,2-dimethyl-3,5-octanedianato)" in Encyclopedia of Reagents for Organic Synthesis, 2004. John Wiley & Sons, Ltd. doi:10.1002/047084289X.rn00449