| MINDY3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MINDY3, C10orf97, CARP, DERP5, MST126, my042, MSTP126, FAM188A, Fam188a, family with sequence similarity 188 member A, MINDY lysine 48 deubiquitinase 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 611649 MGI: 1914210 HomoloGene: 11478 GeneCards: MINDY3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Gene
Fam188a is a protein found in humans. It is also known as Derp5 (Dermal Papilla Derived Protein 5,) c10orf97, or brain my042 protein. It is encoded by the Derp5 gene located on chromosome 10p13.[5][6] Fam188a and its paralogs in other species are all members of the DUF4205 superfamily of protein domains. Fam188a is a highly conserved gene found in all vertebrates. Fam188a is a gene expressed throughout the body.[7]
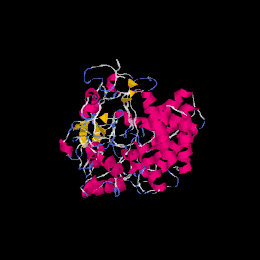 Predicted protein structure of Fam188a using I-TASSER
Predicted protein structure of Fam188a using I-TASSER
Homology
All mammalian species compared share a 95-99% homology similarity to Homo sapiens Fam188a. The reasons behind this are unknown, but it can be inferred that Fam188a must play an important role in proper cellular function in mammals, as even small changes over time in important genes can drastically alter their function.
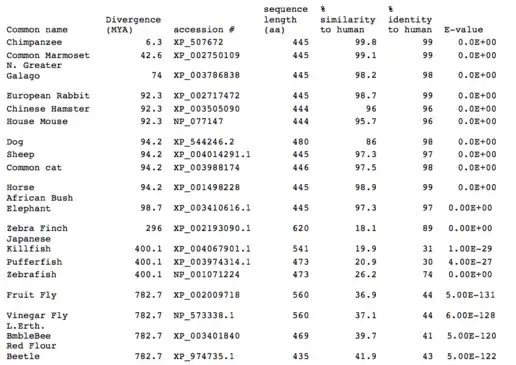 Evolutionary comparison of different species' Fam188a
Evolutionary comparison of different species' Fam188a
Avians, Fish, and Insects also shared a similarity in the 30-40% range with Homo sapiens' Fam188a. Even though there are several hundred million years of divergence from Humans and insects, the fact that there is still a 40% similarity in insects seems to suggest that this gene hasn't changed extremely over such long timespans, and must have been important for other species as well long ago. The splice patterns in these other species aren't known, but in humans there are at least 19 different isoforms but with only 1 major paralog: Fam188b.[8] This paralog differs structurally in 3 exons with Fam188a.
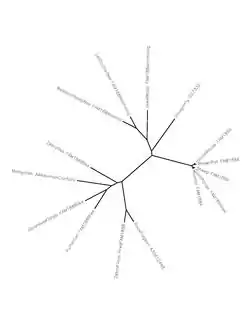 An evolutionary tree between various species that have homologs of Fam188a.
An evolutionary tree between various species that have homologs of Fam188a. Intron/exon map of Fam188a in Homo sapiens
Intron/exon map of Fam188a in Homo sapiens
Protein
Fam188a is a 445-Amino Acid chain. Structurally, it doesn't show many interesting regions that could easily set it apart from other proteins. Fam188a has no transmembrane domains, it has no sequence repeats.
 Fam188a post-translational predicted protein secondary structure
Fam188a post-translational predicted protein secondary structure Fam188a predicted transcription factor binding sites
Fam188a predicted transcription factor binding sites
Function and interacting proteins
The I-TASSER[9] protein folding results show that Fam188a's protein product has a similar structure to 1CFF,[10] a Calmodulin binding peptide. Calmodulin is involved in cell apoptosis as well, so this can shed light on the theorized function of Fam188a as well.
The protein fold is most functionally similar to 3LEW,[11] a “SusD-like carbohydrate binding protein from Bacteroides vulgatus” (Joint Center for Structural Genomics). 3LEW shares a 90% similarity with Fam188a, and although not enough to make a concrete connection, it is enough to establish a base idea of related structures. Another protein that is functionally similar (81%) is 3SNX, a “putative SusD-like carbohydrate binding protein from Bacteroides thetaiotaomicron VPI-5482”.[12] The function of Fam188a can be assumed to play a role in cell apoptosis, and that is because it shares structural similarities with other proteins that are involved in this process. Although there is no CARD-domain as would normally be found in an apoptotic peptide, fam188a does share a similar function with 1cffA, a “calmodulin binding peptide of the Ca2+ pump”.[10] Since Calmodulin is known to cause apoptosis throughout various body tissues via the Ca2+ pump[13][14] and 1cffA shares a 26% similarity with Fam188a, I can hypothesize that apoptosis truly is the function of Fam188a. Other molecules similar to Fam188a are 1linA,[15] and 1qx7M, which share 23% and 18% structure similarity respectively, and both of these molecules deal with either the Ca2+ pump or Calmodulin expression as well.
 Fam188a tissue expression throughout various body tissues
Fam188a tissue expression throughout various body tissues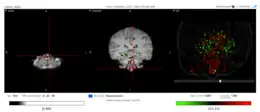 Expression locations of Fam188a in the human brain
Expression locations of Fam188a in the human brain
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000148481 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000026767 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ GeneCard for fam188a
- ↑ HomoloGene: 11478
- ↑ "Fam188a datasheet" (PDF).
- ↑ GeneCard for FAM188B
- ↑ Roy A, Kucukural A, Zhang Y (2010). "I-TASSER: a unified platform for automated protein structure and function prediction". Nature Protocols. 5 (4): 725–38. doi:10.1038/nprot.2010.5. PMC 2849174. PMID 20360767.
- 1 2 PDB: 1CFF; Elshorst B, Hennig M, Försterling H, Diener A, Maurer M, Schulte P, Schwalbe H, Griesinger C, Krebs J, Schmid H, Vorherr T, Carafoli E (1999). "NMR solution structure of a complex of calmodulin with a binding peptide of the Ca2+ pump". Biochemistry. 38 (38): 12320–32. doi:10.1021/bi9908235. PMID 10493800. S2CID 24440343.
- ↑ PDB: 3lew
- ↑ PDB: 3snx
- ↑ Yu W, Niwa T, Miura Y, Horio F, Teradaira S, Ribar TJ, Means AR, Hasegawa Y, Senda T, Niki I (2002). "Calmodulin overexpression causes Ca(2+)-dependent apoptosis of pancreatic beta cells, which can be prevented by inhibition of nitric oxide synthase". Laboratory Investigation. 82 (9): 1229–39. doi:10.1097/01.lab.0000027921.01548.c5. PMID 12218084.
- ↑ Ui-Tei K, Nagano M, Sato S, Miyata Y (2000). "Calmodulin-dependent and -independent apoptosis in cell of a Drosophila neuronal cell line". Apoptosis. 5 (2): 133–40. doi:10.1023/A:1009676528805. PMID 11232241. S2CID 33324141.
- ↑ PDB: 1lin; Vandonselaar M, Hickie RA, Quail JW, Delbaere LT (1994). "Trifluoperazine-induced conformational change in Ca(2+)-calmodulin". Nature Structural Biology. 1 (11): 795–801. doi:10.1038/nsb1194-795. PMID 7634090. S2CID 13431074.



