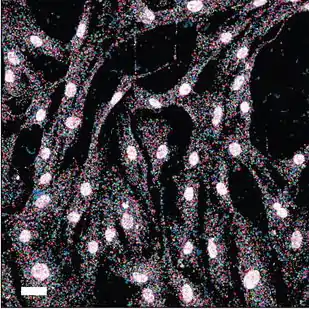
Fluorescent in situ sequencing (FISSEQ) is a method of sequencing a cell's RNA while it remains in tissue or culture using next-generation sequencing.
Introduction
FISSEQ combines the spatial context of RNA-FISH and the global transcriptome profiling of RNA-seq.[1] FISSEQ preserves the tissue allowing single molecule in situ RNA localization. The foundation of the method is a novel nucleic acid sequencing library construction method that stably cross-links cDNA amplicons within biological samples.[2] Sequencing data is then generated through an intensive interleaved microscopy and biochemistry protocol and subsequent image processing and bioinformatics. FISSEQ is compatible with diverse sample types including cell culture, tissue sections, and whole mount embryos. FISSEQ is an example of an extremely dense form of in-situ nucleic acid readout: every letter along the RNA chain is read. Thus, barcodes for FISSEQ can be packed into a short string of DNA, as short as 15-20 nucleotides long for the mouse brain or 5 nucleotides for targeted cancer gene panels.
Methods
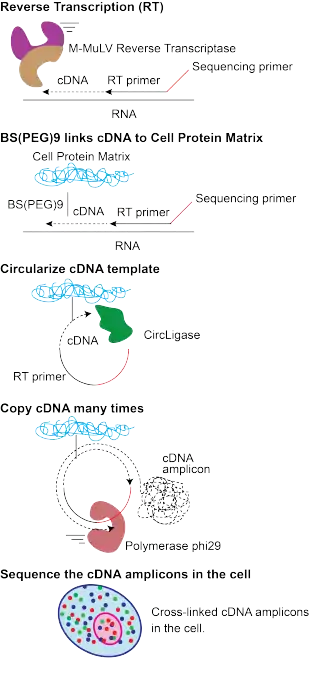
In FISSEQ, a series of biochemical processing steps, such as DNA ligations or single-base DNA polymerase extensions, are performed on a block of fixed tissue, interlaced with fluorescent imaging steps. The process is conceptually identical to the mechanism of fluorescent sequencing by synthesis in a commercial bulk DNA sequencing machine, except that it is performed in fixed tissue. Each DNA or RNA molecule in the sample is first “amplified” (i.e., copied) in-situ via rolling-circle amplification to create a localized “rolling circle colony” (rolony) consisting of identical copies of the parent molecule. A series of biochemical steps are then carried out. In the kth cycle, a fluorescent tag is introduced, the color of which corresponds to the identity of the kth base along the rolony’s parent DNA strand. The system is then “paused” in this state for imaging. The entire sample can be imaged in each cycle. The fluorescent tags are then cleaved and washed away, and the next cycle is initiated. Each rolony – corresponding to a single “parent” DNA or RNA molecule in the tissue – thus appears across a series of fluorescent images, as a localized “spot” with a sequence of colors corresponding to the nucleotide sequence of the parent molecule. The nucleotide sequence of each DNA or RNA molecule is thus read out in-situ via fluorescent microscopy.
History
The development of FISSEQ began over a decade ago.[1] The underlying principles are similar to those that ushered in the sequencing revolution.[3][4][5] In both bulk high-throughput sequencing and FISSEQ, short sequences are locally amplified and then imaged one nucleotide at a time. However, the requirement to sequence RNA in intact tissue—rather than isolated and purified DNA, as in conventional bulk sequencing—posed additional challenges. These limitations were overcome,[2] and FISSEQ allows the joint, high-throughput readout of sequence and spatial information.
See also
References
- 1 2 Mitra RD, Shendure J, Olejnik J, Edyta-Krzymanska-Olejnik, Church GM (2003). "Fluorescent in situ sequencing on polymerase colonies" (PDF). Analytical Biochemistry. 320 (1): 55–65. CiteSeerX 10.1.1.69.5582. doi:10.1016/S0003-2697(03)00291-4. PMID 12895469.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, Terry R, Jeanty SS, Li C, Amamoto R, Peters DT, Turczyk BM, Marblestone AH, Inverso SA, Bernard A, Mali P, Rios X, Aach J, Church GM (2014). "Highly multiplexed subcellular RNA sequencing in situ". Science. 343 (6177): 1360–1363. Bibcode:2014Sci...343.1360L. doi:10.1126/science.1250212. PMC 4140943. PMID 24578530.
- ↑ Church, G. M.; Kieffer-Higgins, S. (1988-04-08). "Multiplex DNA sequencing". Science. 240 (4849): 185–188. Bibcode:1988Sci...240..185C. doi:10.1126/science.3353714. ISSN 0036-8075. PMID 3353714.
- ↑ Bentley, David R.; Balasubramanian, Shankar; Swerdlow, Harold P.; Smith, Geoffrey P.; Milton, John; Brown, Clive G.; Hall, Kevin P.; Evers, Dirk J.; Barnes, Colin L. (2008-11-06). "Accurate whole human genome sequencing using reversible terminator chemistry". Nature. 456 (7218): 53–59. Bibcode:2008Natur.456...53B. doi:10.1038/nature07517. ISSN 1476-4687. PMC 2581791. PMID 18987734.
- ↑ Peters, Brock A.; Kermani, Bahram G.; Sparks, Andrew B.; Alferov, Oleg; Hong, Peter; Alexeev, Andrei; Jiang, Yuan; Dahl, Fredrik; Tang, Y. Tom (2012-07-12). "Accurate whole-genome sequencing and haplotyping from 10 to 20 human cells". Nature. 487 (7406): 190–195. Bibcode:2012Natur.487..190P. doi:10.1038/nature11236. ISSN 1476-4687. PMC 3397394. PMID 22785314.