Fluorobenzaldehyde is a group of three constitutional isomers of fluorinated benzaldehyde.
Properties
The isomers differ in the location of the fluorine, but they have the same chemical formulas.
| Fluorobenzaldehyde isomers | |||
|---|---|---|---|
| Name | o-Fluorobenzaldehyde | m-Fluorobenzaldehyde | p-Fluorobenzaldehyde |
| Structure | 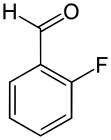 |
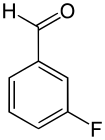 |
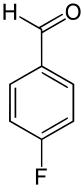 |
| Systematic name | 2-Fluorobenzaldehyde | 3-Fluorobenzaldehyde | 4-Fluorobenzaldehyde |
| Molecular formula | C7H5FO | C7H5FO | C7H5FO |
| Molar mass | 124.11 g/mol | 124.11 g/mol | 124.11 g/mol |
| CAS number | 446-52-6 | 456-48-4 | 459-57-4 |
| EC number | 207-171-2 | 207-266-9 | 459-57-4 |
| Properties | |||
| Melting point | -44.5°C | -10°C | |
| Boiling point | 175°C | 173°C | 181°C |
| Flash point | 55°C | 56°C | 56°C |
| Density | 1.18 g/cm3 | 1.174 g/cm3 | 1.175 g/cm3 |
Preparation
The 4-fluorobenzaldehyde isomer can be produced by a halogen-exchange reaction with 4-chlorobenzaldehyde.[1]
Uses
Fluorobenzaldehyde can be used as a synthetic intermediate because the fluorine can be replaced via oxidation reaction.[1] Due to the aldehyde group, the fluorobenzaldehydes can be used to make a variety of schiff base compounds through a condensation reaction. Schiff bases containing halogenated aromatic rings exhibit antimicrobial properties.[2][3]
References
- 1 2 Yoshida, Yasuo; Kimura, Yoshikazu (1989-08-01). "An improved and practical synthesis of 4-fluorobenzaldehyde by halogen-exchange fluorination reaction". Journal of Fluorine Chemistry. 44 (2): 291–298. doi:10.1016/S0022-1139(00)83946-9. ISSN 0022-1139.
- ↑ Singh, Kiran (2006). "Antibacterial Co(II), Ni(II), Cu(II) and Zn(II) Complexes of Schiff bases Derived from Fluorobenzaldehyde and Triazoles". Journal of Enzyme Inhibition and Medicinal Chemistry. 21 (5): 557–562. doi:10.1080/14756360600642131. ISSN 1475-6366. PMID 17194027. S2CID 38700429.
- ↑ Patel, Ishwar J.; Parmar, Shailesh J. (2010). "Synthesis and Studies of Novel Optically Active Schiff's Base Derivatives and their Antimicrobial Activities". e-Journal of Chemistry. 7 (2): 617–623. doi:10.1155/2010/956242. ISSN 0973-4945.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.