 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| |
| |
| Properties | |
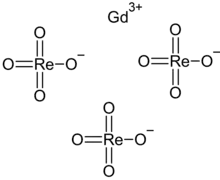
| Gd(ReO4)3 | |
| Solubility | soluble in water and ethanol[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gadolinium perrhenate is an inorganic compound, with the chemical formula of Gd(ReO4)3. It can be obtained by dissolving an excess of gadolinium oxide in a perrhenic acid solution (240 g/L) in the presence of hydrogen peroxide, from which the hydrates are precipitated.[2] Its tetrahydrate loses water by heating to obtain the anhydrous form,[3] which then decomposes at high temperatures to generate gadolinium oxide and rhenium heptoxide.[2]
References
- ↑ Plyushchev, V. E.; Varfolomeev, M. B. Anhydrous perrhenates of rare earth metals(in Russian). Zhurnal Prikladnoi Khimii (Sankt-Peterburg, Russian Federation), 1968. 41 (8): 1643-1646. ISSN: 0044-4618.
- 1 2 Varfolomeev, M. B.; Plyushchev, V. E. Europium and gadolinium perrhenates(in Russian). Zhurnal Neorganicheskoi Khimii, 1967. 12 (2): 353-358. ISSN: 0044-457X.
- ↑ Varfolomeev, M. B.; Ivanova, E. D.; Lunk, Kh. I.; Hilmer, W.; Shamrai, N. B. Thermal stability of rare earth element perrhenate tetrahydrates (Ln(ReO4)3.4H2O)(in Russian). Zhurnal Neorganicheskoi Khimii, 1984. 29 (12): 2995-2998. ISSN: :0044-457X.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.