 | |
| Names | |
|---|---|
| IUPAC name
tetrabromogermane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.270 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Br4Ge | |
| Molar mass | 392.246 g·mol−1 |
| Appearance | Colorless solid |
| Density | 2.123 g/cm3 |
| Melting point | 26 °C (79 °F; 299 K) |
| Boiling point | 185.9 °C (366.6 °F; 459.0 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
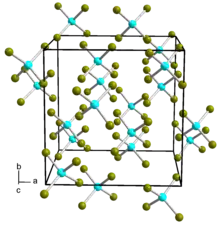
| Structure[1] | |
| α-Cubic (SnI4 type) β-Monoclinic (SnBr4 type) | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
83.3 kcal/mol |
| Related compounds | |
Other anions |
Germanium tetrafluoride Germanium tetrachloride Germanium tetraiodide |
Other cations |
Carbon tetrabromide Silicon tetrabromide Tin(IV) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Germanium tetrabromide is the inorganic compound with the formula GeBr4. It is a colorless solid that melts near room temperature. It can be formed by treating solid germanium with bromine, or by treating a germanium-copper mixture with bromine:[2]
- Ge + Br2 → GeBr4
From this reaction, GeBr4 has a heat of formation of 83.3 kcal/mol.[3]
The compound is liquid at 25 °C, and forms an interlocking liquid structure.[4] From room temperature down to −60 °C the structure takes on a cubic α form, whereas at lower temperatures it takes on a monoclinic β form.
References
- ↑ Köhler, J.; Okudera, Η.; Simon, A. (2005). "Crystal structure of germanium tetrabromide, β-GeBr4, low temperature modification". Zeitschrift für Kristallographie - New Crystal Structures. Walter de Gruyter GmbH. 220 (1–4): 554. doi:10.1524/ncrs.2005.220.14.554. ISSN 2197-4578.
- ↑ P. W. Schenk (1963). "Silicon and Germanium". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2page=718. NY, NY: Academic Press.
- ↑ Evans, D. F.; Richards, R. E. (1952). "233. The heats of formation of germanium tetrabromide and germanium tetraiodide". Journal of the Chemical Society (Resumed). Royal Society of Chemistry (RSC): 1292. doi:10.1039/jr9520001292. ISSN 0368-1769.
- ↑ Swamy, K. N.; Bhuiyan, L. B. (1980). "The Reference Interaction Site Model and the Structure of Liquid Germanium Tetrabromide". Physics and Chemistry of Liquids. Informa UK Limited. 9 (2): 169–174. doi:10.1080/00319108008084774. ISSN 0031-9104.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.