 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| TiBr2 | |
| Molar mass | 207.68 |
| Appearance | black solid |
| Density | 4.41 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
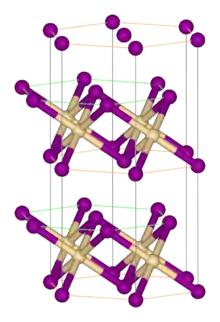
Titanium(II) bromide is the inorganic compound with the formula TiBr2. It is a black micaceous solid. It adopts the cadmium iodide structure, featuring octahedral Ti(II) centers. It arises via the reaction of the elements:[1]
- Ti + Br2 → TiBr2
The compound reacts with caesium bromide to give the linear chain compound CsTiBr3.[2]
References
- ↑ Klemm, Wilhelm; Grimm, Ludwig (1942). "Zur Kenntnis der Dihalogenide des Titans und Vanadins". Zeitschrift für Anorganische und Allgemeine Chemie. 249 (2): 198–208. doi:10.1002/zaac.19422490204.
- ↑ Meyer, G.; Hinz, D. J.; Flörke, U. (1993). "Crystal structure of caesium titanium tribromide, CsTiBr3". Zeitschrift für Kristallographie - Crystalline Materials. 208 (2): 370. Bibcode:1993ZK....208..370M. doi:10.1524/zkri.1993.208.Part-2.370.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.