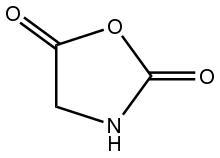
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Oxazolidine-2,5-dione | |
| Other names
glycine N-carboxyanhydride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.016.882 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H3NO3 | |
| Molar mass | 101.061 g·mol−1 |
| Appearance | white solid |
| Density | 1.74 g/cm3[1] |
| Melting point | 96–98[2] °C (205–208 °F; 369–371 K) |
| Hazards | |
| GHS labelling:[3] | |
  | |
| Danger | |
| H315, H318, H335 | |
| P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P354+P338, P317, P319, P321, P332+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Glycine N-carboxyanhydride is an organic compound with the formula HNCH(CO)2O. A colorless solid, it is the product of phosgenation (reaction with phosgene) of glycine.[4][5] Glycine N-carboxyanhydride is the simplest member of the amino acid N-carboxyanhydrides. It is also the parent of the 2,5-oxazolidinedione family of heterocycles.
Other derivatives
2,5-Oxazolidinediones can also be prepared from Schiff base derivatives of amino acids.[6]
See also
- 2,4-Oxazolidinedione, parent ring found in a variety anticonvulsant drugs.
References
- ↑ Kanazawa, Hitoshi; Matsuura, Yoshiki; Tanaka, Nobuo; Kakudo, Masao; Komoto, Tadashi; Kawai, Tohru (1976). "The Crystal and Molecular Structure ofN-Carboxy Anhydride of Glycine". Bulletin of the Chemical Society of Japan. 49 (4): 954–956. doi:10.1246/bcsj.49.954.
- ↑ Wilder, Renee; Mobashery, Shahriar (1992). "The use of triphosgene in preparation of N-carboxy .alpha.-amino acid anhydrides". The Journal of Organic Chemistry. 57 (9): 2755–2756. doi:10.1021/jo00035a044.
- ↑ "Oxazolidine-2,5-dione". pubchem.ncbi.nlm.nih.gov. Retrieved 5 April 2022.
- ↑ Kricheldorf HR (September 2006). "Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides". Angewandte Chemie. 45 (35): 5752–84. doi:10.1002/anie.200600693. PMID 16948174.
- ↑ Tian ZY, Zhang Z, Wang S, Lu H (October 2021). "A Moisture-Tolerant Route to Unprotected α/β-Amino Acid N-carboxyanhydrides and Facile Synthesis of Hyperbranched Polypeptides". Nature Communications. 12 (1): 5810. Bibcode:2021NatCo..12.5810T. doi:10.1038/s41467-021-25689-y. PMC 8490447. PMID 34608139.
- ↑ Sucu BO, Ocal N, Erden I (2015). "Direct synthesis of imidazolidin-4-ones via cycloadditions of imines with a Leuchs' anyhdride". Tetrahedron Letters. 56 (20): 2590–2. doi:10.1016/j.tetlet.2015.04.002.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.