| GYG1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | GYG1, GSD15, GYG, glycogenin 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 603942 MGI: 1351614 HomoloGene: 31219 GeneCards: GYG1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Glycogenin-1 is an enzyme that is involved in the biosynthesis of glycogen. It is capable of self-glucosylation, forming an oligosaccharide primer that serves as a substrate for glycogen synthase. This is done through an inter-subunit mechanism. It also plays a role in glycogen metabolism regulation. Recombinant human glycogenin-1 was expressed in E. coli and purified using conventional chromatography techniques.[5]
Glycogen metabolism
Glycogen is a multi-branched polysaccharide. It is primary means of glucose storage in animal cells. In the human body, the two main tissues which store glycogen are liver and skeletal muscle.[6] Glycogen is typically more concentrated in the liver, but because humans have much more muscle mass, our muscles store about three quarters of the total glycogen in our body.
Location of glycogen
The function of liver glycogen is to maintain glucose homeostasis, generating glucose via glycogenolysis to compensate for the decrease of glucose levels that can occur between meals. Thanks to the presence of the glucose-6-phosphatase enzyme, the hepatocytes are capable of turning glycogen to glucose, releasing it into blood to prevent hypoglycemia.
In skeletal muscle, glycogen is used as an energy source for muscle contraction during exercise. The different functions of glycogen in muscle or liver make the regulation mechanisms of its metabolism differ in each tissue.[7] These mechanisms are based mainly on the differences on structure and on the regulation of the enzymes that catalyze synthesis, glycogen synthase (GS), and degradation, glycogen phosphorylase (GF).
Glycogen synthesis
Glycogenin is the initiator of the glycogen biosynthesis.[8][9] This protein is a glycosyl transferase that have the ability of autoglycosilation using UDP-glucose,[10] which helps in the growth of itself until forming an oligosaccharide made by 8 glucoses. Glycogenin is an oligomer, and it's capable of interacting with several proteins. In recent years, a family of proteins has been identified, the GNIPs (glycogenin-interacting protein), that interacts with glycogenin stimulating its autoglycolsilation activity.
Glycogenin-1
In humans, two isoforms of glycogenin can be expressed: glycogenin-1, with a molecular weight of 37 kDa and codified by GYG1 gen, which is expressed mostly in muscles; and glycogenin-2, with a molecular weight of 66 kDa and codified by GYG2 gen, which is expressed mainly in liver, cardiac muscle and other types of tissue, but not in skeletal muscle.[11] Glycogenin-1 was described by analyzing the glycogen of skeletal muscle. It was determined that this molecule was united by a covalent bond to each mature molecule of muscular glycogen.[12]
Gene
Structure
The glycogenin-1 gene, which spans over 13kb, consists of seven exons and six introns. Its proximal promoter contains a TATA box, a cyclic AMP responsive element, and two putative Sp1 binding sites in a CpG island, a DNA region with a high frequency of CpG sites. There are also nine E-boxes that bind the basic helix-loop-helix of muscle-specific transcription factors.[13]
Location and transcription
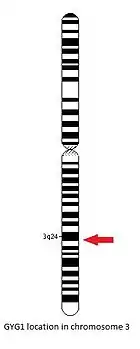
The GYG1 gene is located on the long arm of the chromosome 3, between positions 24 and 25, from base pair 148,709,194 to base pair 148,745,455.[14]
Transcription of human glycogenin-1 is mainly initiated at 80bp and 86bp upstream the translator’s codon beginning. Transcriptions factors have different binding sites for its development, some examples are: GATA, activator protein 1 and 2 (AP-1 and AP-2), and numerous potential Octamer-1 binding sites.[15]
Deficiency
A Glycogenin-1 deficiency leads to Glycogen storage disease type XV.
Mutation
Deficiency of glycogenin-1 is detected in the sequence of the glycogenin-1 gene, GYG1, which revealed a non-sense mutation in one allele and a missense mutation, Thr83Met, in the other. The missense mutation resulted in inactivation of the autoglycosylation of glycogenin-1, which is necessary for the priming of glycogen synthesis in muscle. Autoglycosylation of glycogenin-1 occurs at Tyr195 by a gulose-1-O-tyrosine linkage. An induced missense mutation of this residue results in inactivated autoglycosylation. However, missense mutation affecting some other residues of glycogenin-1 has also been shown to eliminate autoglycosilation.
Consequences
The phenotypic features of the skeletal muscle in a patient with this disorder are muscle glycogen depletion, mitochondrial proliferation, and a marked predominance of slow-twitch, oxidative muscle fibres. The mutations in the glycogenin-1 gene GYG1 are also a cause of cardiomyopathy and arrhythmia.[11]
See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000163754 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000019528 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Details for Glycogenin-1, 1-333 aa, Recombinant Protein. .
- ↑ Alonso MD, Lomako J, Lomako WM, Whelan WJ (June 1995). "Catalytic activities of glycogenin additional to autocatalytic self-glucosylation". The Journal of Biological Chemistry. 270 (25): 15315–9. doi:10.1074/jbc.270.25.15315. PMID 7797519.
- ↑ Newsholme EA and Start C. (1973). In Regulation in Metabolism, J. Wiley & sons. eds. London Chapter 3.
- ↑ Lomako J, Lomako WM, Whelan WJ (December 1988). "A self-glucosylating protein is the primer for rabbit muscle glycogen biosynthesis". FASEB Journal. 2 (15): 3097–103. doi:10.1096/fasebj.2.15.2973423. PMID 2973423. S2CID 24083688.
- ↑ Viskupic E, Cao Y, Zhang W, Cheng C, DePaoli-Roach AA, Roach PJ (December 1992). "Rabbit skeletal muscle glycogenin. Molecular cloning and production of fully functional protein in Escherichia coli". The Journal of Biological Chemistry. 267 (36): 25759–63. doi:10.1016/S0021-9258(18)35674-6. PMID 1281472.
- ↑ Skurat AV, Roach PJ (January 1996). "Multiple mechanisms for the phosphorylation of C-terminal regulatory sites in rabbit muscle glycogen synthase expressed in COS cells". The Biochemical Journal. 313 ( Pt 1) (Pt 1): 45–50. doi:10.1042/bj3130045. PMC 1216907. PMID 8546708.
- 1 2 Moslemi AR, Lindberg C, Nilsson J, Tajsharghi H, Andersson B, Oldfors A (April 2010). "Glycogenin-1 deficiency and inactivated priming of glycogen synthesis". The New England Journal of Medicine. 362 (13): 1203–10. doi:10.1056/NEJMoa0900661. PMID 20357282.
- ↑ Zhai L, Dietrich A, Skurat AV, Roach PJ (January 2004). "Structure-function analysis of GNIP, the glycogenin-interacting protein". Archives of Biochemistry and Biophysics. 421 (2): 236–42. doi:10.1016/j.abb.2003.11.017. PMID 14984203.
- ↑ van Maanen MH, Fournier PA, Palmer TN, Abraham LJ (July 1999). "Characterization of the human glycogenin-1 gene: identification of a muscle-specific regulatory domain". Gene. 234 (2): 217–26. doi:10.1016/s0378-1119(99)00211-5. PMID 10395894.
- ↑ "Where is the GYG1 gene located?" Archived 2020-09-20 at the Wayback Machine.
- ↑ van Maanen M, Fournier PA, Palmer TN, Abraham LJ (October 1999). "Characterization of mouse glycogenin-1 cDNA and promoter region". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1447 (2–3): 284–90. doi:10.1016/s0167-4781(99)00159-1. PMID 10542328.
External links
- van Maanen M, Fournier PA, Palmer TN, Abraham LJ (October 1999). "Characterization of mouse glycogenin-1 cDNA and promoter region". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1447 (2–3): 284–90. doi:10.1016/s0167-4781(99)00159-1. PMID 10542328.
- van Maanen MH, Fournier PA, Palmer TN, Abraham LJ (July 1999). "Characterization of the human glycogenin-1 gene: identification of a muscle-specific regulatory domain". Gene. 234 (2): 217–26. doi:10.1016/s0378-1119(99)00211-5. PMID 10395894.