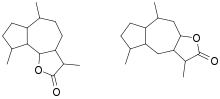
In organic chemistry, a guaianolide is a type of sesquiterpene lactone consisting of a gamma-lactone and either a cyclopentane or cyclopentene, both fused to a central cycloheptane or cycloheptene structure. There are two subclasses, structural isomers differing in the location that part of the lactone is bonded to the central ring, known as 6,12-guaianolides and 8,12-guaianolides.[1]
Because some of the natural products in this class of tricyclic phytochemical have been found to be potentially biologically active, there has been interest in their chemical syntheses.[2] The full biosynthetic origin of most of the known guaianolides has not been established, but the pathway is generally presumed to begin with the formation of a germacrene lactone derived from farnesyl pyrophosphate.[1]
References
- 1 2 Henrik Toft Simonsen, Corinna Weitzel and Søren Brøgger Christensen (2013). K. G. Ramawat, J. M. Merillon (ed.). "Guaianolide Sesquiterpenoids: Pharmacology and Biosynthesis". Natural Products: 3069–3098. doi:10.1007/978-3-642-22144-6_134. ISBN 978-3-642-22143-9.
- ↑ Hu, Xirui; Musacchio, Andrew J.; Shen, Xingyu; Tao, Yujia; Maimone, Thomas J. (2019). "Allylative Approaches to the Synthesis of Complex Guaianolide Sesquiterpenes from Apiaceae and Asteraceae". J. Am. Chem. Soc. 141 (37): 14904–14915. doi:10.1021/jacs.9b08001. PMC 6818654. PMID 31448610.