 | |
| Names | |
|---|---|
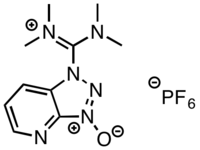
| IUPAC name
1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.103.434 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H15F6N6OP | |
| Molar mass | 380.235 g·mol−1 |
| Appearance | White crystalline solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
HATU (Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium) is a reagent used in peptide coupling chemistry to generate an active ester from a carboxylic acid. HATU is used along with Hünig's base (N,N-diisopropylethylamine, DIPEA), or triethylamine to form amide bonds. Typically DMF is used as solvent, although other polar aprotic solvents can also be used.[1]
History
HATU was first reported by Louis A. Carpino in 1993 as an efficient means of preparing active esters derived from 1-hydroxy-7-azabenzotriazole (HOAt).[2] HATU can exist as either the uronium salt (O-form) or the less reactive iminium salt (N-form). HATU was initially reported as the O-form using the original preparation reported by Carpino; however, X-ray crystallographic and NMR studies revealed the true structure of HATU to be the less reactive guanidinium isomer.[3] It is, however, possible to obtain the uronium isomer by preparing HATU using KOAt in place of HOAt and working up the reaction mixture quickly to prevent isomerisation.
Reactions
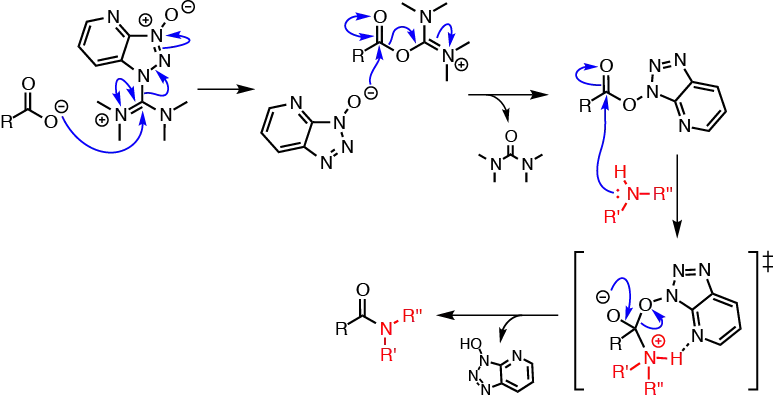
HATU is commonly encountered in amine acylation reactions (i.e., amide formation). Such reactions are typically performed in two distinct reaction steps: (1) reaction of a carboxylic acid with HATU to form the OAt-active ester; then (2) addition of the nucleophile (amine) to the active ester solution to afford the acylated product.
The reaction mechanism of carboxylic acid activation by HATU and subsequent N-acylation is summarised in the figure below. The mechanism is shown using the more commonly encountered and commercially available iminium isomer; a similar mechanism, however, is likely to apply to the uronium form. In the first step, the carboxylate anion (formed by deprotonation by an organic base [not shown]) attacks HATU to form the unstable O-acyl(tetramethyl)isouronium salt. The OAt anion rapidly attacks the isouronium salt, affording the OAt-active ester and liberating a stoichiometric quantity of tetramethylurea. Addition of a nucleophile, such as an amine, to the OAt-active ester results in acylation.
The high coupling efficiencies and fast reaction rates associated with HATU coupling are thought to arise from a neighbouring group effect brought about by the pyridine nitrogen atom, which stabilises the incoming amine through a hydrogen-bonded 7-membered cyclic transition state.[4]
Because of the extraordinary coupling efficiency of HATU, it has often been used for intramolecular amidation (coupling of a carboxylic acid and an amine of the same molecule). For example, the formation of cyclo-tetrapeptides through the head-to-tail reaction of linear tetrapeptides assisted by HATU has been reported.[5]
References
- ↑ "Amine to Amide (Coupling) - HATU".
- ↑ Carpino, Louis A (1993). "1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive". Journal of the American Chemical Society. 115 (10): 4397–4398. doi:10.1021/ja00063a082.
- ↑ Carpino, Louis A; Imazumi, Hideko; El-Faham, Ayman; Ferrer, Fernando J; Zhang, Chongwu; Lee, Yunsub; Foxman, Bruce M; Henklein, Peter; Hanay, Christiane; Mügge, Clemens; Wenschuh, Holger; Klose, Jana; Beyermann, Michael; Bienert, Michael (2002). "The Uronium/Guanidinium Peptide Coupling Reagents: Finally the True Uronium Salts". Angewandte Chemie International Edition. 41 (3): 441–445. doi:10.1002/1521-3773(20020201)41:3<441::AID-ANIE441>3.0.CO;2-N. PMID 12491372.
- ↑ Carpino, Louis A; Imazumi, Hideko; Foxman, Bruce M; Vela, Michael J; Henklein, Peter; El-Faham, Ayman; Klose, Jana; Bienert, Michael (2000). "Comparison of the Effects of 5- and 6-HOat on Model Peptide Coupling Reactions Relative to the Cases for the 4- and 7-Isomers†,‡". Organic Letters. 2 (15): 2253–2256. doi:10.1021/ol006013z. PMID 10930256.
- ↑ Müntener, Thomas; Thommen, Fabienne; Joss, Daniel; Kottelat, Jérémy; Prescimone, Alessandro; Häussinger, Daniel (16 April 2019). "Synthesis of chiral nine and twelve-membered cyclic polyamines from natural building blocks". Chemical Communications. 55 (32): 4715–4718. doi:10.1039/C9CC00720B. ISSN 1364-548X.