 | |
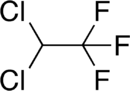
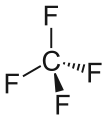 Chemical structures of dichlorotrifluoroethane (top) and tetrafluormethane (bottom), the two major components of Halotron I | |
| Hazards | |
|---|---|
| GHS labelling: | |
  | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration) |
3.2% (4 hrs, inhalation) |
| Safety data sheet (SDS) | Halotron |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Halotron I is a fire extinguishing agent based on the raw material HCFC-123 (93%) mixed with tetrafluoromethane and argon as propellants.
Global emission concerns
It was originally introduced in 1992 to replace the severely ozone-depleting Halon 1211 (bromochlorodifluoromethane). Halon 1211 has a global warming potential of 2,070,[2] whereas Halotron I's GWP is 77, a 96 percent reduction.[3]
Performance
In December 2011, Halotron I was tested against "hidden fires", spurred by the effectiveness its predecessor demonstrated on an in-flight fire aboard a Delta L-1011 flight on March 17, 1991. The test was conducted at UL, and demonstrated similar effectiveness as Halon 1211, with significantly less human and global harm.[4] Although the fire extinguishing effectiveness is similar, Halotron I requires a larger chemical volume to get the same ratings as Halon 1211.
DOT classification
UN1956, Compressed Gases, N.O.S., Nonflammable Gas. IMCO CLASS: 2.2
References
- ↑ "Halotron". www.halotron.com. Retrieved 24 April 2023.
- ↑ Hodnebrog, Øivind; Åmås, Borgar; Fuglestvedt, Jan; Marston, George; Myhre, Gunnar; Nielsen, Claus Jørgen; Sandstad, Marit; Shine, Keith P.; Wallington, Tim J. (July 9, 2020). "Updated Global Warming Potentials and Radiative Efficiencies of Halocarbons and Other Weak Atmospheric Absorbers". Reviews of Geophysics. 58 (3).
- ↑ "Sixth Triennial International Fire & Cabin Safety Research Conference" (PDF). fire.tc.faa.gov. 2010. Retrieved 24 April 2023.
- ↑ "R0201336.pdf" (PDF). NIST.gov. Retrieved December 23, 2017.
