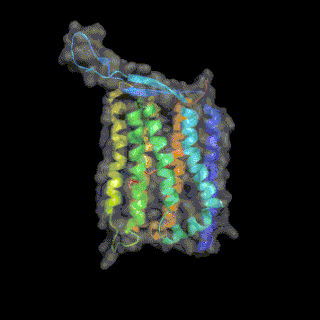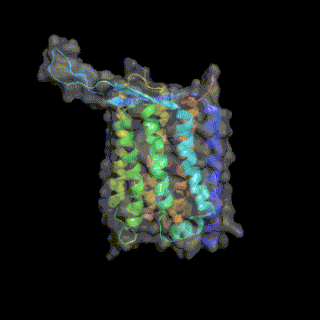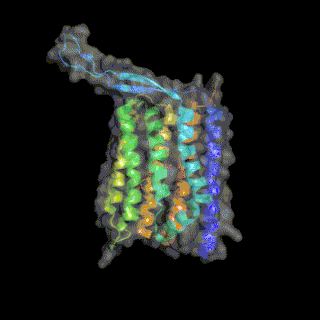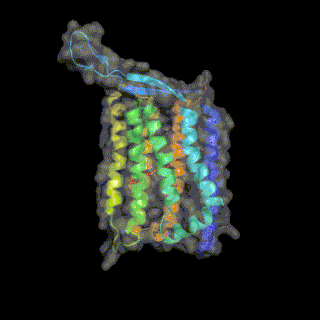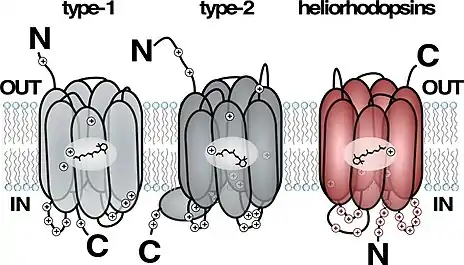
Heliorhodopsin is a family of rhodopsins discovered in 2018 by Alina Pushkarev in the laboratory of Professor Oded Beja.[1] The new family of heliorhodopsins has a distinct protein sequence from known Type 1 (microbial) and Type 2 (animal) rhodopsins. Heliorhodopsins also exhibit the reverse orientation in the membrane compared with the other rhodopsins, with the N-terminus facing the inside of the cell and the C-terminus outside the cell.[1]
Heliorhodopsins use all-trans retinal as a chromophore, and do not have any ion pumping activity across the membrane. Heliorhodopsins are distributed globally and exist in eukaryotes, prokaryotes and even some viruses.[1] Despite the wide distribution, Heliorhodopsins are never present in true diderms, where there is a proper double membrane around the microorganism. It has been suggested that the function of Heliorhodopsin requires a direct interaction with the environment.[2]
Crystal structures of Heliorhodopsins suggest they form a homodimer, contain a fenestration leading toward the retinal molecule and have a large extracellular loop facing the outside of the cell.[3][4][5]
References
- 1 2 3 Pushkarev, Alina; Inoue, Keiichi; Larom, Shirley; Flores-Uribe, José; Singh, Manish; Konno, Masae; Tomida, Sahoko; Ito, Shota; Nakamura, Ryoko; Tsunoda, Satoshi P.; Philosof, Alon (June 2018). "A distinct abundant group of microbial rhodopsins discovered using functional metagenomics". Nature. 558 (7711): 595–599. doi:10.1038/s41586-018-0225-9. ISSN 0028-0836. PMID 29925949. S2CID 49330919.
- ↑ Flores‐Uribe, José; Hevroni, Gur; Ghai, Rohit; Pushkarev, Alina; Inoue, Keiichi; Kandori, Hideki; Béjà, Oded (June 2019). "Heliorhodopsins are absent in diderm (Gram‐negative) bacteria: Some thoughts and possible implications for activity". Environmental Microbiology Reports. 11 (3): 419–424. doi:10.1111/1758-2229.12730. ISSN 1758-2229. PMID 30618066. S2CID 58666386.
- ↑ Shihoya, Wataru; Inoue, Keiichi; Singh, Manish; Konno, Masae; Hososhima, Shoko; Yamashita, Keitaro; Ikeda, Kento; Higuchi, Akimitsu; Izume, Tamaki; Okazaki, Sae; Hashimoto, Masanori (October 2019). "Crystal structure of heliorhodopsin". Nature. 574 (7776): 132–136. doi:10.1038/s41586-019-1604-6. ISSN 0028-0836. PMID 31554965. S2CID 202760270.
- ↑ Kovalev, K.; Volkov, D.; Astashkin, R.; Alekseev, A.; Gushchin, I.; Haro-Moreno, J. M.; Rogachev, A.; Balandin, T.; Borshchevskiy, V.; Popov, A.; Bourenkov, G. (2019-09-12). "High Resolution Structural Insights into Heliorhodopsin Family". bioRxiv: 767665. doi:10.1101/767665.
- ↑ Lu, Yang; Zhou, X. Edward; Gao, Xiang; Wang, Na; Xia, Ruixue; Xu, Zhenmei; Leng, Yu; Shi, Yuying; Wang, Guangfu; Melcher, Karsten; Xu, H. Eric (January 2020). "Crystal structure of heliorhodopsin 48C12". Cell Research. 30 (1): 88–90. doi:10.1038/s41422-019-0266-0. ISSN 1001-0602. PMC 6951262. PMID 31879417.