 | |
 | |
| Names | |
|---|---|
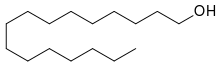
| Preferred IUPAC name
Hexadecan-1-ol | |
| Other names
Cetanol, Cetyl alcohol, Ethal, Ethol, Hexadecanol, Hexadecyl alcohol, Palmityl alcohol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.048.301 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H34O | |
| Molar mass | 242.447 g·mol−1 |
| Appearance | White crystals or flakes |
| Odor | Very faint, waxy |
| Density | 0.811 g/cm3 |
| Melting point | 49.3 °C (120.7 °F; 322.4 K) |
| Boiling point | 344 °C (651 °F; 617 K) |
| Insoluble | |
| Solubility | Very soluble in ether, benzene, and chloroform. Soluble in acetone. Slightly soluble in alcohol. |
| log P | 7.25[2] |
| Acidity (pKa) | 16.20 |
| −183.5·10−6 cm3/mol | |
Refractive index (nD) |
1.4283 (79 °C) |
| Viscosity | 53 cP (75 °C) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 185 °C (365 °F; 458 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
5000 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Cetyl alcohol /ˈsiːtəl/, also known as hexadecan-1-ol and palmityl alcohol, is a C-16 fatty alcohol with the formula CH3(CH2)15OH. At room temperature, cetyl alcohol takes the form of a waxy white solid or flakes. The name cetyl derives from the whale oil (cetacea oil, from Latin: cetus, lit. 'whale', from Ancient Greek: κῆτος, romanized: kētos, lit. 'huge fish')[3] from which it was first isolated.[4]
Preparation
Cetyl alcohol was discovered in 1817 by the French chemist Michel Chevreul when he heated spermaceti, a waxy substance obtained from sperm whale oil, with caustic potash (potassium hydroxide). Flakes of cetyl alcohol were left behind on cooling.[5] Modern production is based around the chemical reduction of ethyl palmitate.[6]
Occurrence and uses
The ether chimyl alcohol, derived from cetyl alcohol and glycerol, is a component of some lipid membranes.
Cetyl alcohol is used in the cosmetic industry as an opacifier in shampoos, or as an emollient, emulsifier or thickening agent in the manufacture of skin creams and lotions.[7] It is also employed as a lubricant for nuts and bolts, and is the active ingredient in some "liquid pool covers" (forming a non-volatile surface layer to reduce water evaporation, related latent vaporization heat loss, and thus to retain heat in the pool). Moreover, it can also be used as a non-ionic co-surfactant in emulsion applications.[8]
Side effects
People who have eczema can be sensitive to cetyl alcohol,[9][10] though this may be due to impurities rather than cetyl alcohol itself.[11] However, cetyl alcohol is sometimes included in medications used for the treatment of eczema.[12]
Related compounds
References
- ↑ Merck Index, 11th Edition, 2020.
- ↑ "Hexadecan-1-ol_msds".
- ↑ M. Raneft, D.; Eaker, H.; W. Davis, R. (2001). "A guide to the pronunciation and meaning of cetacean taxonomic names" (PDF). Aquatic Mammals. 27 (2): 185. Archived from the original (PDF) on 2016-03-27. Retrieved 2020-04-26.
- ↑ Nordegren, Thomas (2002). The A-Z Encyclopedia of Alcohol and Drug Abuse. Universal Publishers. p. 165. ISBN 1-58112-404-X.
- ↑ Booth, James Curtis (1862). The Encyclopedia of Chemistry, Practical and Theoretical. Philadelphia, H.C. Baird. p. 429.
- ↑ "Cetyl alcohol". Encyclopedia Britannica. July 20, 1998. Retrieved 2023-01-28.
- ↑ Smolinske, Susan C (1992). Handbook of Food, Drug, and Cosmetic Excipients. CRC Press. pp. 75–76. ISBN 0-8493-3585-X.
- ↑ Golemanov, Konstantin; Tcholakova, Slavka; Denkov, Nikolai D.; Gurkov, Theodor (April 2006). "Selection of surfactants for stable paraffin-in-water dispersions, undergoing solid−liquid transition of the dispersed particles". Langmuir. 22 (8): 3560–3569. doi:10.1021/la053059y. ISSN 0743-7463. PMID 16584227.
- ↑ Gaul, LE (1969). "Dermatitis from cetyl and stearyl alcohols". Archives of Dermatology. 99 (5): 593. doi:10.1001/archderm.1969.01610230085016. PMID 4238421.
- ↑ Soga, F; Katoh, N; Kishimoto, S (2004). "Contact dermatitis due to lanoconazole, cetyl alcohol and diethyl sebacate in lanoconazole cream". Contact Dermatitis. 50 (1): 49–50. doi:10.1111/j.0105-1873.2004.00271j.x. PMID 15059111. S2CID 19854024.
- ↑ Komamura, H; Doi, T; Inui, S; Yoshikawa, K (1997). "A case of contact dermatitis due to impurities of cetyl alcohol". Contact Dermatitis. 36 (1): 44–6. doi:10.1111/j.1600-0536.1997.tb00921.x. PMID 9034687. S2CID 23444831.
- ↑ Kato N; Numata T; Kanzaki T (1987). "Contact dermatitis due to Japanese pharmacopeia cetyl alcohol". Skin Research. 29 (suppl 3): 258–262.
