Hexagonal ferrites or hexaferrites are a family of ferrites with hexagonal crystal structure. The most common member is BaFe12O19, also called barium ferrite, BaM, etc. BaM is a strong room-temperature ferrimagnetic material with high anisotropy along the c axis.[1] All the hexaferrite members are constructed by stacking a few building blocks in a certain order.
Basic building blocks






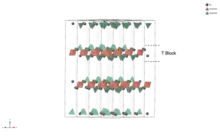


S block
The S block is very common in hexaferrites, which has a chemical formula of MeS6O82+. MeS are smaller metal cations, for example, Fe and other transition metals[2] or noble metals.[3] The S block is essentially a slab cut along the plane of an AB2O4 spinel. Each S block has one A layer and one B layer. The A layer features MeS-centered tetrahedron and MeS-centered octahedron, while the B layer is made up of edge-sharing MeS-centered octahedron. Both A and B layers have the same chemical formula of MeS3O42+.
R block
The R block has a chemical formula of MeLMeS6O112-. MeL are larger metal cations, for example, alkaline earth metals (Ba,[4] Sr,[5]) rare earth metals,[5] Pb,[6] etc. The point group symmetry of the R block is . The large metal cations are located in the middle layer of the three hexagonally packed layers. This block is also composed of face-sharing MeS-centered octahedra and MeS-centered trigonal bipyramids.
T block
The T block has a chemical formula of MeL2MeS8O142-. The point group symmetry of the T block is . One T block consists of 4 oxygen layers with the two MeL atoms substituting two oxygen atoms in the middle two layers. In one T block, there are both MeS-centered octahedra and MeS-centered tetrahedra.
Family nembers
M-type ferrite
M-type ferrite is made up of alternating S and R blocks in the sequence of SRS*R*. (* denotes rotating that layer around the c axis by 180°.) The chemical formula of M-type ferrite is MeLMeS12O19. Common examples are BaFe12O19, SrFe12O19. It exhibits space group symmetry. For BaFe12O19, a = 5.89 Å and c = 23.18 Å.[7] M-ferrite is a very robust ferrimagnetic material, thus widely used as fridge magnets, card strips, magnets in speakers, magnetic material in linear tape-open.

W-type ferrite
W-type ferrite, like the M-type, consists of S and R blocks, but the stacking order and the number of blocks are different. The stacking sequence in a W-ferrite is SSRS*S*R* and its chemical formula is MeLMeS18O27. It exhibits space group symmetry. One example of W-type ferrite is BaFe18O27, with a = 5.88 Å and c = 32.85 Å.[8]
R-type ferrite
R-type ferrite has a chemical formula of MeLMeS6O11 with a space group of . Unlike other hexaferrites, R-type ferrite doesn't have an S block. Instead, it only has single B layers extracted from the S block. The stacking sequence is BRB*R*.
Y-type ferrite
Y-type ferrite has a chemical formula of MeL2MeS14O22 with a space group of . One example is Ba2Co2Fe12O22 with a = 5.86 Å and c = 43.5 Å.[9] Y-type ferrite is built up with S and T blocks with an order of 3(ST) in one unit cell. There is no horizontal mirror plane in a Y-type ferrite.
Z-type ferrite
Z-type ferrite has a chemical formula of MeL3MeS26O41 with a space group of . It has a complicated stacking of SRSTS*R*S*T* in one unit cell. Some Z-type members may have sophisticated magnetic properties along different directions.[10] One example is Ba3Co2Fe24O41 with a = 5.88 Å and c = 52.3 Å.
X-type ferrite
X-type ferrite has a chemical formula of MeL2MeS30O46 with a space group of . The stacking order is 3(SRS*S*R*) in one unit cell. One example is Sr2Co2Fe28O46 with c = 83.74 Å.[11]
References
- ↑ Pullar, Robert C. (2012-09-01). "Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics". Progress in Materials Science. 57 (7): 1191–1334. doi:10.1016/j.pmatsci.2012.04.001. ISSN 0079-6425.
- ↑ Ishiwata, Shintaro; Terasaki, Ichiro; Azuma, Masaki; Takano, Mikio (2008-05-01). "High pressure synthesis and structure of a new magnetoplumbite-type cobalt oxide SrCo12O19". Journal of Solid State Chemistry. 181 (5): 1273–1278. Bibcode:2008JSSCh.181.1273I. doi:10.1016/j.jssc.2008.02.024. ISSN 0022-4596.
- ↑ Foo, M. L.; Huang, Q.; Lynn, J. W.; Lee, Wei-Li; Klimczuk, Tomasz; Hagemann, I. S.; Ong, N. P.; Cava, R. J. (2006-02-01). "Synthesis, structure and physical properties of Ru ferrites: BaRu5O11 (M=Li and Cu) and BaM′2Ru4O11 (M′=Mn, Fe and Co)". Journal of Solid State Chemistry. 179 (2): 563–572. doi:10.1016/j.jssc.2005.11.014. ISSN 0022-4596.
- ↑ Nemrava, Sandra; Vinnik, Denis A.; Hu, Zhiwei; Valldor, Martin; Kuo, Chang-Yang; Zherebtsov, Dmitry A.; Gudkova, Svetlana A.; Chen, Chien-Te; Tjeng, Liu Hao; Niewa, Rainer (2017-04-03). "Three Oxidation States of Manganese in the Barium Hexaferrite BaFe12–xMnxO19". Inorganic Chemistry. 56 (7): 3861–3866. doi:10.1021/acs.inorgchem.6b02688. ISSN 0020-1669. PMID 28290672.
- 1 2 Li, Jun; Medina, Elena A.; Stalick, Judith K.; Sleight, Arthur W.; Subramanian, M. A. (2016-05-01). "Structural studies of CaAl12O19, SrAl12O19, La2/3+δAl12–δO19, and CaAl10NiTiO19 with the hibonite structure; indications of an unusual type of ferroelectricity". Zeitschrift für Naturforschung B. 71 (5): 475–484. doi:10.1515/znb-2015-0224. ISSN 1865-7117. S2CID 102258634.
- ↑ Comer, J.J.; Croft, W.J.; Kestigian, M.; Carter, J.R. (March 1967). "An x-ray and electron microscope study of the compound PbAl12O19". Materials Research Bulletin. 2 (3): 293–302. doi:10.1016/0025-5408(67)90013-X.
- ↑ Ounnunkad, S.; Winotai, P. (2006-06-01). "Properties of Cr-substituted M-type barium ferrites prepared by nitrate–citrate gel-autocombustion process". Journal of Magnetism and Magnetic Materials. 301 (2): 292–300. Bibcode:2006JMMM..301..292O. doi:10.1016/j.jmmm.2005.07.003. ISSN 0304-8853.
- ↑ Braun, P. B. (1957). "The crystal structures of a new group of ferromagnetic compounds". Philips Res. Rep. 12: 491–548.
- ↑ Shin, H. S.; Kwon, S.-J. (June 1993). "X-ray powder diffraction patterns of two Y-type hexagonal ferrites". Powder Diffraction. 8 (2): 98–101. Bibcode:1993PDiff...8...98S. doi:10.1017/s0885715600017905. ISSN 0885-7156. S2CID 94413989.
- ↑ Pullar, Robert C. (2012-09-01). "Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics". Progress in Materials Science. 57 (7): 1191–1334. doi:10.1016/j.pmatsci.2012.04.001. ISSN 0079-6425.
- ↑ Wu, Meixia (2020). "Single-Crystal Growth and Room-Temperature Magnetocaloric Effect of X-Type Hexaferrite Sr2Co2Fe28O46". Inorganic Chemistry. 59 (10): 6755–6762. doi:10.1021/acs.inorgchem.9b03724. OSTI 1631933. PMID 32364708. S2CID 218504683.