Holotomography (HT) is a laser technique to measure the three-dimensional refractive index (RI) tomogram of a microscopic sample such as biological cells and tissues. Because the RI can serve as an intrinsic imaging contrast for transparent or phase objects, measurements of RI tomograms can provide label-free quantitative imaging of microscopic phase objects. In order to measure 3-D RI tomogram of samples, HT employs the principle of holographic imaging and inverse scattering. Typically, multiple 2D holographic images of a sample are measured at various illumination angles, employing the principle of interferometric imaging. Then, a 3D RI tomogram of the sample is reconstructed from these multiple 2D holographic images by inversely solving light scattering in the sample.
History
The first theoretical proposal was presented by Emil Wolf,[1] and the first experimental demonstration was shown by Fercher et al.[2] From 2000s, HT techniques had been extensively studied and applied to the field of biology and medicine, by several research groups including the MIT spectroscopy laboratory. Both the technical developments and applications of HT have been significantly advanced. In 2012 the first commercial HT company Nanolive[3] was founded, later followed by Tomocube in 2014.
Principles
The principle of HT is very similar to X-ray computed tomography (CT) or CT scan. CT scan measures multiple 2-D X-ray images of a human body at various illumination angles, and a 3-D tomogram (X-ray absorptivity) is then retrieved via the inverse scattering theory. Both the X-ray CT and laser HT shares the same governing equation – Helmholtz equation, the wave equation for a monochromatic wavelength. HT is also known as optical diffraction tomography.[4]
Advantages and limitations
HT provides following advantages over conventional 3D microscopic techniques.
- Label-free: Cellular membrane and subcellular organelles can clearly be imaged without using exogenous labeling agents. Thus, there are no issues of phototoxicity, photobleaching and photodamaging.
- Quantitative imaging capability: HT directly measures cell’s 3D RI maps, which is intrinsic optical properties of materials. Because the measured RI can be translated into the mass density of a cell and using this information, mass of a cell can also be retrieved.
- Precise and fast measurements: HT provides the spatial resolution down to approximately 100 nm and the temporal resolution of a few to a hundred frames per second, depending on the numerical apertures of used objective lenses and the speed of an image sensor.
However, 3D RI tomography does not provide molecular specificity. Generally, the measured RI information cannot be directly related to information about molecules or proteins, except for notable cases such as gold nanoparticles[5] or lipid droplets[6] that exhibit distinctly high RI values compared to cell cytoplasm.
Applications
The applications of HT include:[7]
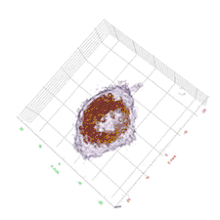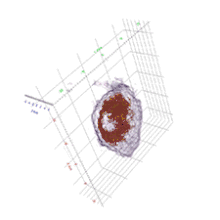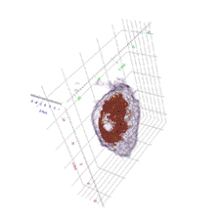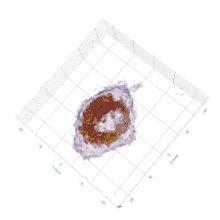
Cell biology
HT provides 3D dynamic images of live cells and thin tissues without using exogenous labeling agents such as fluorescence proteins or dyes. HT enables quantitative live cell imaging, and also provides quantitative information such as cell volume, surface area, protein concentration. The label-free imaging and quantification of chromosomes were presented.[8] The regulatory pathway of proteasome degradation by autophagy in cells were studies using HT.[9]
Correlative imaging
HT can be used with other imaging modalities for correlative imaging. For example, a combination of HT and fluorescence imaging enables a synergistic analytic approach.[10][11] HT provides structural information whereas fluorescence signal provides molecular specific imaging, an optical analogous to positron emission tomography (PET) and CT. Various approaches have been reported for correlative imaging approaches using HT.
Lipid quantification
Intracellular lipid droplets play important roles in energy storage and metabolism, and are also related to various pathologies, including cancer, obesity, and diabetes mellitus. HT enables label-free and quantitative imaging and analysis for free or intracellular lipid droplets. Because lipid droplets have distinctly high RI (n > 1.375) compared to other parts of cytoplasm, the measurements of RI tomograms provide information about the volume, concentration, and dry mass of lipid droplets.[12] Recently, HT was used to evaluate the therapeutic effects of a nanodrug designed to affect the targeted delivery of lobeglitazone by measuring lipid droplets in foam cells.[13]
Experimental laboratory
HT provide various quantitative imaging capability, providing morphological, biochemical, and mechanical properties of individuals cells. 3D RI tomography directly provides morphological properties including volume, surface area, and sphericity (roundness) of a cell. Local RI value can be translated into biochemical information or cytoplasmic protein concentration, because the RI of a solution is linearly proportional to its concentration.[14] In particular, for the case of red blood cells, RI value can be converted into hemoglobin concentration. Measurements of dynamic cell membrane fluctuation, which can also be obtained with a HT instrument, provides information about cellular deformability. Furthermore, these various quantitative parameters can be obtained at the single cell level, allowing correlative analysis between various cellular parameters. HT has been utilized for the study of red blood cells,[15] white blood cells,[16] blood storage,[17] and diabetes.[18]
Infectious diseases
The quantitative label-free imaging capability of HT have been exploited for the study of various infectious diseases. In particular, parasites-invaded host cells can be effectively imaged and studied using HT. This is because the staining or labeling of parasites requires complicated preparation process and the staining/labeling is not very effective in several parasites. The invasion of plasmodium falciparum, or malaria inducing parasites, to individual red blood cells were measured using HT.[19] The structural and biophysical alteration to host cells and parasites have been systematically analyzed. The invasion of babesia parasites to red blood cells were also studied.[20] Toxoplasma gondii, an apicomplexan parasite causing toxoplasmosis, can infect nucleated cells. The alterations of 3D morphology and biophysical properties of T gondii infected cells were studied using HT.[21]
Biotechnology
The cell volume and dry mass of individual bacteria or micro algae can be effectively quantified using HT.[22] Because it does not require the staining process while providing the precise quantification values, HT can be used for testing the efficacy of engineered stains.
Scientific community
The following are active scientific conferences on HT, as a part of quantitative phase imaging techniques:
- Quantitative phase imaging conference, SPIE Photonics West
The HT technique and applications have been included in the following special issues of scientific journals:
- Special Issue on Quantitative Phase Imaging for Label-Free Cytometry in Cytometry Part A, 2019
- Research Topic on Quantitative Phase Imaging and Its Applications to Biophysics, Biology, and Medicine in Frontiers in Physics
See also
References
- ↑ Wolf, Emil (1969). "Three-dimensional structure determination of semi-transparent objects from holographic data". Optics Communications. 1 (4): 153–156. Bibcode:1969OptCo...1..153W. doi:10.1016/0030-4018(69)90052-2.
- ↑ Fercher, A.F.; Bartelt, H.; Becker, H.; Wiltschko, E. (1979). "Image formation by inversion of scattered field data: experiments and computational simulation". Applied Optics. 18 (14): 2427–39. Bibcode:1979ApOpt..18.2427F. doi:10.1364/AO.18.002427. PMID 20212679.
- ↑ "Home". nanolive.ch. Retrieved 2020-08-26.
- ↑ Lauer, V (2002). "New approach to optical diffraction tomography yielding a vector equation of diffraction tomography and a novel tomographic microscope". Journal of Microscopy. 205 (2): 165–176. doi:10.1046/j.0022-2720.2001.00980.x. PMID 11879431. S2CID 30791056.
- ↑ Kim, Doyeon (2016). "Label-free high-resolution 3-D imaging of gold nanoparticles inside live cells using optical diffraction tomography". bioRxiv 10.1101/097113.
- ↑ Kim, Kyoohyun (2016). "Three-dimensional label-free imaging and quantification of lipid droplets in live hepatocytes". Scientific Reports. 6: 36815. arXiv:1611.01774. Bibcode:2016NatSR...636815K. doi:10.1038/srep36815. PMC 5118789. PMID 27874018.
- ↑ Park, YongKeun (2018). "Quantitative phase imaging in biomedicine". Nature Photonics. 12 (10): 578–589. Bibcode:2018NaPho..12..578P. doi:10.1038/s41566-018-0253-x. PMID 26648557. S2CID 126144855.
- ↑ Kim, Seul (2020). "PRMT6-mediated H3R2me2a guides Aurora B to chromosome arms for proper chromosome segregation". Nature Communications. 11 (1): 612. doi:10.1038/s41467-020-14511-w. PMC 6992762. PMID 32001712.
- ↑ Choi, Won Hoon (11 August 2020). "Aggresomal sequestration and STUB1-mediated ubiquitylation during mammalian proteaphagy of inhibited proteasomes". PNAS. 117 (32): 19190–19200. doi:10.1073/pnas.1920327117. PMC 7430983. PMID 32723828.
- ↑ Kim, Y. S.; Lee, S.; Jung, J.; Shin, S.; Choi, H. G.; Cha, G. H.; Park, W.; Lee, S.; Park, Y. (2018). "Combining Three-Dimensional Quantitative Phase Imaging and Fluorescence Microscopy for the Study of Cell Pathophysiology". Yale J Biol Med. 91 (3): 267–277. PMC 6153632. PMID 30258314.
- ↑ Lambert, Aubrey (2020). "Live Cell Imaging with Holotomography and Fluorescence". Microscopy Today. 28: 18–23. doi:10.1017/S1551929519001032.
- ↑ Kim, Kyoohyun; Lee, Seoeun; Yoon, Jonghee; Heo, Jihan; Choi, Chulhee; Park, Yongkeun (2016). "Three-dimensional label-free imaging and quantification of lipid droplets in live hepatocytes". Scientific Reports. 6: 36815. arXiv:1611.01774. Bibcode:2016NatSR...636815K. doi:10.1038/srep36815. PMC 5118789. PMID 27874018.
- ↑ Park, Sangwoo; Ahn, Jae Won; Jo, Youngju; Kang, Ha-Young; Kim, Hyun Jung; Cheon, Yeongmi; Kim, Jin Won; Park, Yongkeun; Lee, Seongsoo; Park, Kyeongsoon (2020). "Label-Free Tomographic Imaging of Lipid Droplets in Foam Cells for Machine-Learning-Assisted Therapeutic Evaluation of Targeted Nanodrugs". ACS Nano. 14 (2): 1856–1865. doi:10.1021/acsnano.9b07993. PMID 31909985. S2CID 210087144.
- ↑ Baber, R. (1952). "Interference microscopy and mass determination". Nature. 169 (4296): 366–7. Bibcode:1952Natur.169..366B. doi:10.1038/169366b0. PMID 14919571. S2CID 4188525.
- ↑ Park, YongKeun (2010). "Measurement of red blood cell mechanics during morphological changes". PNAS. 107 (15): 6731–6. Bibcode:2010PNAS..107.6731P. doi:10.1073/pnas.0909533107. PMC 2872375. PMID 20351261.
- ↑ Yoon, Jonghee (2015). "Label-free characterization of white blood cells by measuring 3D refractive index maps". Biomedical Optics Express. 6 (10): 3865–75. arXiv:1505.02609. Bibcode:2015arXiv150502609Y. doi:10.1364/BOE.6.003865. PMC 4605046. PMID 26504637.
- ↑ Park, Hyunjoo (2016). "Measuring cell surface area and deformability of individual human red blood cells over blood storage using quantitative phase imaging". Scientific Reports. 6: 34257. Bibcode:2016NatSR...634257P. doi:10.1038/srep34257. PMC 5048416. PMID 27698484.
- ↑ Lee, SangYun (2017). "Refractive index tomograms and dynamic membrane fluctuations of red blood cells from patients with diabetes mellitus". Scientific Reports. 7 (1): 1039. Bibcode:2017NatSR...7.1039L. doi:10.1038/s41598-017-01036-4. PMC 5430658. PMID 28432323.
- ↑ Park, YongKeun (2008). "Refractive index maps and membrane dynamics of human red blood cells parasitized by Plasmodium falciparum". PNAS. 105 (37): 13730–13735. Bibcode:2008PNAS..10513730P. doi:10.1073/pnas.0806100105. PMC 2529332. PMID 18772382.
- ↑ HyunJoo, Park (2015). "Characterizations of individual mouse red blood cells parasitized by Babesia microti using 3-D holographic microscopy". Scientific Reports. 5: 10827. arXiv:1505.00832. Bibcode:2015NatSR...510827P. doi:10.1038/srep10827. PMC 4650620. PMID 26039793.
- ↑ Firdaus, Egy Rahman; Park, Ji‐Hoon; Lee, Seong‐Kyun; Park, Yongkeun; Cha, Guang‐Ho; Han, Eun‐Taek (2020). "3D morphological and biophysical changes in a single tachyzoite and its infected cells using three-dimensional quantitative phase imaging". Journal of Biophotonics. 13 (8): e202000055. doi:10.1002/jbio.202000055. PMID 32441392. S2CID 218831871.
- ↑ Ahn, Jung Ho; Seo, Hogyun; Park, Woojin; Seok, Jihye; Lee, Jong An; Kim, Won Jun; Kim, Gi Bae; Kim, Kyung-Jin; Lee, Sang Yup (2020). "Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase". Nature Communications. 11 (1): 1970. doi:10.1038/s41467-020-15839-z. PMC 7181634. PMID 32327663.