 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Hydroxypropan-2-one | |
| Systematic IUPAC name
1-Hydroxyacetone | |
| Other names
1-Hydroxy-2-propanone Acetomethyl alcohol Acetol | |
| Identifiers | |
3D model (JSmol) |
|
| 605368 | |
| ChemSpider | |
| ECHA InfoCard | 100.003.750 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H6O2 | |
| Molar mass | 74.079 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Sweet |
| Density | 1.059 g/cm3[1] |
| Melting point | −17 °C (1 °F; 256 K) |
| Boiling point | 145–146 °C (293–295 °F; 418–419 K) |
| Vapor pressure | 7.5 hPa at 20 °C[2] |
Refractive index (nD) |
1.415[1] |
| Hazards | |
| GHS labelling: | |
| H226[2] | |
| Flash point | 56 °C (closed cup)[2] |
| Explosive limits | Upper limit: 14.9%(V) Lower limit: 3%(V)[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2200 mg/kg (rat, oral)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
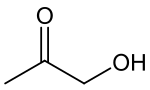
Hydroxyacetone, also known as acetol, is the organic chemical with the formula CH3C(O)CH2OH. It consists of a primary alcohol substituent on acetone. It is an α-hydroxyketone, also called a ketol, and is the simplest hydroxy ketone structure. It is a colorless, distillable liquid.
Preparation
It is produced commercially by dehydration of glycerol.[4]
Hydroxyacetone is commercially available, but it also may be synthesized on a laboratory scale by a substitution reaction on bromoacetone.[5]
Reactions
It undergoes rapid polymerization, including forming a hemiacetal cyclic dimer. Under alkaline conditions, it undergoes a rapid aldol condensation.
Hydroxyacetone can be produced by degradation of various sugars. In foods, it is formed by the Maillard reaction. It reacts further to form other compounds with various aromas.[6] As such it finds some use as a flavoring.
See also
- Acyloin, the simplest secondary α-hydroxy ketone.
References
- 1 2 Nodzu, Ryuzaburo (1935). "On the Action of Phosphate Upon Hexoses. I. The Formation of Acetol From Glucose in Acidic Solution of Potassium Phosphate". Bull. Chem. Soc. Jpn. 10 (3): 122–130. doi:10.1246/bcsj.10.122.
- 1 2 3 4 Sigma-Aldrich Co., Hydroxyacetone. Retrieved on 2 July 2015.
- ↑ Smyth, H. F. Jr; Carpenter, C. P. (January 1948). "Further experience with the range finding test in the industrial toxicology laboratory". The Journal of Industrial Hygiene and Toxicology. 30 (1): 63–8. PMID 18895731.
- ↑ Carl J. Sullivan, Anja Kuenz, Klaus‐Dieter Vorlop (2018). "Propanediols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_163.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ↑ Levene, P. A.; Walti, A. (1930). "Acetol". Org. Synth. 10: 1. doi:10.15227/orgsyn.010.0001.
- ↑ Nursten, Harry E. (1998). "The Mechanism of Formation of 3-Methylcyclopent-2-en-2-olone". In O'Brien, J.; Nursten, H. E.; Crabbe, M. J.; Ames, J. M. (eds.). The Maillard Reaction in Foods and Medicine. Elsevier. pp. 65–68. ISBN 9781845698447.
External links
 Media related to Hydroxyacetone at Wikimedia Commons
Media related to Hydroxyacetone at Wikimedia Commons