Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.
Hydroxymethylation with formaldehyde
A common method for hydroxymethylation involves the reaction of formaldehyde with active C-H and N-H bonds:
- R3C-H + CH2O → R3C-CH2OH
- R2N-H + CH2O → R2N-CH2OH
A typical active C-H bond is provided by a terminal acetylene[1] or the alpha protons of an aldehyde.[2] In industry, hydroxymethylation of acetaldehyde with formaldehyde is used in the production of pentaerythritol:
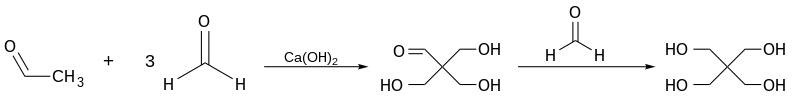
P-H bonds are also prone to reaction with formaldehyde. Tetrakis(hydroxymethyl)phosphonium chloride ([P(CH2OH)4]Cl) is produced in this way from phosphine (PH3).[3]
Hydroxymethylation in demethylation
5-Methylcytosine is a common epigenetic marker. The methyl group is modified by oxidation of the methyl group in a process called hydroxymethylation:[4]
- RCH3 + O → RCH2OH
This oxidation is thought to be a prelude to removal, regenerating cytosine.
Representative reactions
A two-step hydroxymethylation of aldehydes involves methylenation followed by hydroboration-oxidation:[5]
- RCHO + Ph3P=CH2 → RCH=CH2 + Ph3PO
- RCH=CH2 + R2BH → RCH2-CH2BR2
- RCH2-CH2BR2 + H2O2 → RCH2-CH2OH + "HOBR2"
Silylmethyl Grignard reagents are nucleophilic reagents for hydroxymethylation of ketones:[6]
- R2C=O + ClMgCH2SiR'3 → R2C(OMgCl)CH2SiR'3
- R2C(OMgCl)CH2SiR'3 + H2O + H2O2 → R2C(OH)CH2OH + "HOSiR'3"
Reactions of hydroxymethylated compounds
A common reaction of hydroxymethylated compounds is further reaction with a second equivalent of an active X-H bond:
- hydroxymethylation: X-H + CH2O → X-CH2OH
- crosslinking: X-H + X-CH2OH → X-CH2-X + H2O
This pattern is illustrated by the use of formaldehyde in the production various polymers and resins from phenol-formaldehyde condensations (Bakelite, Novolak, and calixarenes). Similar crosslinking occurs in urea-formaldehyde resins.
The hydroxymethylation of N-H and P-H bonds can often be reversed by base. This reaction is illustrated by the preparation of tris(hydroxymethyl)phosphine:[7]
- [P(CH2OH)4]Cl + NaOH → P(CH2OH)3 + H2O + H2C=O + NaCl
When conducted in the presence of chlorinating agents, hydroxymethylation leads to chloromethylation as illustrated by thee Blanc chloromethylation.
Related
- Hydroxyethylation involves the installation of the CH2CH2OH group, as practiced in ethoxylation.
- Aminomethylation is often effected with Eschenmoser's salt, [(CH3)2NCH2]OTf[8]
References
- ↑ John Hooz; Jorge Cabezas; Sergio Musmanni; Jose Calzada (1990). "Propargylation of Alkyl Halides: (E)-6,10-Dimethyl-5,9-Undecadien-1-Yne and (E)-7,11-Dimethyl-6,10-Dodecadien-2-yn-1-ol". Organic Syntheses. 69: 120. doi:10.15227/orgsyn.069.0120.
- ↑ Robert K. Boeckman Jr; Douglas J. Tusch; Kyle F. Biegasiewicz (2015). "Organocatalyzed Direct Asymmetric α-Hydroxymethylation of Aldehydes". Organic Syntheses. 92: 320–327. doi:10.15227/orgsyn.092.0320.
- ↑ Svara, Jürgen; Weferling, Norbert; Hofmann, Thomas (2006). "Phosphorus Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_545.pub2. ISBN 978-3527306732.
- ↑ Greco, Carolina M.; Kunderfranco, Paolo; Rubino, Marcello; Larcher, Veronica; Carullo, Pierluigi; Anselmo, Achille; Kurz, Kerstin; Carell, Thomas; Angius, Andrea; Latronico, Michael V. G.; Papait, Roberto; Condorelli, Gianluigi (2016). "DNA hydroxymethylation controls cardiomyocyte gene expression in development and hypertrophy". Nature Communications. 7: 12418. Bibcode:2016NatCo...712418G. doi:10.1038/ncomms12418. PMC 4976219. PMID 27489048.
- ↑ Eric J. Leopold (1986). "Selective Hydroboration of a 1,3,7-Triene: Homogeraniol". Organic Syntheses. 64: 164. doi:10.15227/orgsyn.064.0164.
- ↑ Kohei Tamao; Neyoshi Ishida; Yoshihiko Ito; Makoto Kumada (1990). "Nucleophilic Hydroxymethylation by the (Isopropoxydimethylsilyl)Methyl Grignard Reagent: 1-(Hydroxymethyl)Cyclohexanol from Cyclohexanone". Organic Syntheses. 69: 96. doi:10.15227/orgsyn.069.0096.
- ↑ M. Caporali, L. Gonsalvi, F. Zanobini, M. Peruzzini (2011). Synthesis of the Water-Soluble Bidentate (P,N) Ligand PTN(Me). Inorganic Syntheses. Vol. 35. pp. 92–108. doi:10.1002/9780470651568.ch5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Gaudry, Michel; Jasor, Yves; Khac, Trung Bui (1979). "Regioselective Mannich Condensation with Dimethyl(Methylene)Ammonium Trifluoroacetate: 1-(Dimethylamino)-4-Methyl-3-Pentanone". Organic Syntheses. 59: 153. doi:10.15227/orgsyn.059.0153.