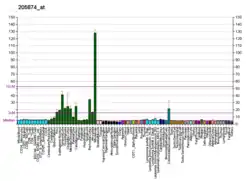
| ITPKA | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ITPKA, IP3-3KA, IP3KA, inositol-trisphosphate 3-kinase A | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 147521 MGI: 1333822 HomoloGene: 1671 GeneCards: ITPKA | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Inositol-trisphosphate 3-kinase A is an enzyme that in humans is encoded by the ITPKA gene.[5][6][7]
Structure
ITPKA is one of three inositol-trisphosphate 3-kinase (ITP3K) genes in humans. ITP3K proteins regulate inositol phosphate metabolism by phosphorylation of the second messenger inositol 1,4,5-trisphosphate to produce Ins(1,3,4,5)P4, which is sometimes abbreviated as IP4. Structurally, ITPKA belongs to the inositol polyphosphate kinase (IPK) family. The activity of the inositol 1,4,5-trisphosphate 3-kinase is responsible for regulating the levels of a large number of inositol polyphosphates that are important in cellular signaling, most notably, inositol trisphosphate, which is the enzyme's only substrate. Both calcium/calmodulin and protein phosphorylation mechanisms control its activity. It is also a substrate for the cyclic AMP-dependent protein kinase, calcium/calmodulin- dependent protein kinase II, and protein kinase C in vitro. ITPKA and ITPKB are 68% identical in the C-terminus region The amino- terminal region of ITPKA binds filamentous actin. This property localizes the ITPKA to dendritic spines in principal neurons.[8][9][10] ITPKA is expressed physiologically in neurons, but it is sometimes expressed in cancer cells and may contribute to processes of metastasis.[11]
Physiological function
ITPKA participates in learning and memory processes in neurons.[12][13]
Roles in human disease
Although ITPKA is expressed physiologically in neurons and testis, it sometimes becomes expressed in cancer cells, and the expression usually makes the cancer more aggressive.[11][14]
Relationship to F-tractin
F-tractin is amino acids 9-52 of rat ITPKA. It was later determined that amino acids 9-40 were sufficient for binding filamentous actin.[15][16] When fused to a reporter, such as green fluorescent protein, It is useful for the visualization of actin dynamics in living cells.[17][18]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000137825 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000027296 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Erneux C, Roeckel N, Takazawa K, Mailleux P, Vassart G, Mattei MG (October 1992). "Localization of the genes for human inositol 1,4,5-trisphosphate 3-kinase A (ITPKA) and B (ITPKB) to chromosome regions 15q14-q21 and 1q41-q43, respectively, by in situ hybridization". Genomics. 14 (2): 546–7. doi:10.1016/S0888-7543(05)80265-4. PMID 1330886.
- ↑ Takazawa K, Perret J, Dumont JE, Erneux C (December 1990). "Human brain inositol 1,4,5-trisphosphate 3-kinase cDNA sequence". Nucleic Acids Research. 18 (23): 7141. doi:10.1093/nar/18.23.7141. PMC 332787. PMID 2175886.
- ↑ "Entrez Gene: ITPKA inositol 1,4,5-trisphosphate 3-kinase A".
- ↑ Yamada M, Kakita A, Mizuguchi M, Rhee SG, Kim SU, Ikuta F (March 1993). "Specific expression of inositol 1,4,5-trisphosphate 3-kinase in dendritic spines". Brain Research. 606 (2): 335–40. doi:10.1016/0006-8993(93)91004-C. PMID 8387863. S2CID 10790958.
- ↑ Schell MJ, Erneux C, Irvine RF (October 2001). "Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus". The Journal of Biological Chemistry. 276 (40): 37537–46. doi:10.1074/jbc.M104101200. PMID 11468283.
- ↑ Windhorst S, Minge D, Bähring R, Hüser S, Schob C, Blechner C, Lin HY, Mayr GW, Kindler S (March 2012). "Inositol-1,4,5-trisphosphate 3-kinase A regulates dendritic morphology and shapes synaptic Ca2+ transients". Cellular Signalling. 24 (3): 750–7. doi:10.1016/j.cellsig.2011.11.010. PMID 22120525.
- 1 2 Windhorst S, Fliegert R, Blechner C, Möllmann K, Hosseini Z, Günther T, Eiben M, Chang L, Lin HY, Fanick W, Schumacher U, Brandt B, Mayr GW (February 2010). "Inositol 1,4,5-trisphosphate 3-kinase-A is a new cell motility-promoting protein that increases the metastatic potential of tumor cells by two functional activities". The Journal of Biological Chemistry. 285 (8): 5541–54. doi:10.1074/jbc.M109.047050. PMC 2820782. PMID 20022963.
- ↑ Chung S, Kim IH, Lee D, Park K, Kim JY, Lee YK, Kim EJ, Lee HW, Choi JS, Son GH, Sun W, Shin KS, Kim H (April 2016). "The role of inositol 1,4,5-trisphosphate 3-kinase A in regulating emotional behavior and amygdala function". Scientific Reports. 6: 23757. Bibcode:2016NatSR...623757C. doi:10.1038/srep23757. PMC 4823716. PMID 27053114.
- ↑ Choi B, Lee HW, Mo S, Kim JY, Kim HW, Rhyu IJ, Hong E, Lee YK, Choi JS, Kim CH, Kim H (2018). "Inositol 1,4,5-trisphosphate 3-kinase A overexpressed in mouse forebrain modulates synaptic transmission and mGluR-LTD of CA1 pyramidal neurons". PLOS ONE. 13 (4): e0193859. Bibcode:2018PLoSO..1393859C. doi:10.1371/journal.pone.0193859. PMC 5884490. PMID 29617377.
- ↑ Windhorst S, Song K, Gazdar AF (August 2017). "Inositol-1,4,5-trisphosphate 3-kinase-A (ITPKA) is frequently over-expressed and functions as an oncogene in several tumor types". Biochemical Pharmacology. 137: 1–9. doi:10.1016/j.bcp.2017.03.023. PMC 5555585. PMID 28377279.
- ↑ Johnson HW, Schell MJ (December 2009). "Neuronal IP3 3-kinase is an F-actin-bundling protein: role in dendritic targeting and regulation of spine morphology". Molecular Biology of the Cell. 20 (24): 5166–80. doi:10.1091/mbc.E09-01-0083. PMC 2793293. PMID 19846664.
- ↑ Yi J, Wu XS, Crites T, Hammer JA (March 2012). "Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells". Molecular Biology of the Cell. 23 (5): 834–52. doi:10.1091/mbc.E11-08-0731. PMC 3290643. PMID 22219382.
- ↑ Belin BJ, Goins LM, Mullins RD (2014). "Comparative analysis of tools for live cell imaging of actin network architecture". Bioarchitecture. 4 (6): 189–202. doi:10.1080/19490992.2014.1047714. PMC 4914014. PMID 26317264.
- ↑ Melak M, Plessner M, Grosse R (February 2017). "Actin visualization at a glance". Journal of Cell Science. 130 (3): 525–530. doi:10.1242/jcs.189068. PMID 28082420.
Further reading
- Takazawa K, Perret J, Dumont JE, Erneux C (September 1991). "Molecular cloning and expression of a new putative inositol 1,4,5-trisphosphate 3-kinase isoenzyme". The Biochemical Journal. 278 (Pt 3): 883–6. doi:10.1042/bj2780883. PMC 1151429. PMID 1654894.
- Takazawa K, Erneux C (November 1991). "Identification of residues essential for catalysis and binding of calmodulin in rat brain inositol 1,4,5-trisphosphate 3-kinase". The Biochemical Journal. 280 (Pt 1): 125–9. doi:10.1042/bj2800125. PMC 1130609. PMID 1660262.
- Takazawa K, Perret J, Dumont JE, Erneux C (January 1991). "Molecular cloning and expression of a human brain inositol 1,4,5-trisphosphate 3-kinase". Biochemical and Biophysical Research Communications. 174 (2): 529–35. doi:10.1016/0006-291X(91)91449-M. PMID 1847047.
- Lin AN, Barnes S, Wallace RW (August 1990). "Phosphorylation by protein kinase C inactivates an inositol 1,4,5-trisphosphate 3-kinase purified from human platelets". Biochemical and Biophysical Research Communications. 170 (3): 1371–6. doi:10.1016/0006-291X(90)90546-Y. PMID 2167676.
- Takazawa K, Vandekerckhove J, Dumont JE, Erneux C (November 1990). "Cloning and expression in Escherichia coli of a rat brain cDNA encoding a Ca2+/calmodulin-sensitive inositol 1,4,5-trisphosphate 3-kinase". The Biochemical Journal. 272 (1): 107–12. doi:10.1042/bj2720107. PMC 1149663. PMID 2176078.
- Ryu SH, Lee SY, Lee KY, Rhee SG (November 1987). "Catalytic properties of inositol trisphosphate kinase: activation by Ca2+ and calmodulin". FASEB Journal. 1 (5): 388–93. doi:10.1096/fasebj.1.5.2824270. PMID 2824270. S2CID 26541634.
- Communi D, Vanweyenberg V, Erneux C (April 1997). "D-myo-inositol 1,4,5-trisphosphate 3-kinase A is activated by receptor activation through a calcium:calmodulin-dependent protein kinase II phosphorylation mechanism". The EMBO Journal. 16 (8): 1943–52. doi:10.1093/emboj/16.8.1943. PMC 1169797. PMID 9155020.
- Woodring PJ, Garrison JC (November 1997). "Expression, purification, and regulation of two isoforms of the inositol 1,4,5-trisphosphate 3-kinase". The Journal of Biological Chemistry. 272 (48): 30447–54. doi:10.1074/jbc.272.48.30447. PMID 9374536.
- Schell MJ, Erneux C, Irvine RF (October 2001). "Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus". The Journal of Biological Chemistry. 276 (40): 37537–46. doi:10.1074/jbc.M104101200. PMID 11468283.
- Mishra J, Bhalla US (September 2002). "Simulations of inositol phosphate metabolism and its interaction with InsP(3)-mediated calcium release". Biophysical Journal. 83 (3): 1298–316. Bibcode:2002BpJ....83.1298M. doi:10.1016/S0006-3495(02)73901-5. PMC 1302229. PMID 12202356.
- Dewaste V, Moreau C, De Smedt F, Bex F, De Smedt H, Wuytack F, Missiaen L, Erneux C (August 2003). "The three isoenzymes of human inositol-1,4,5-trisphosphate 3-kinase show specific intracellular localization but comparable Ca2+ responses on transfection in COS-7 cells". The Biochemical Journal. 374 (Pt 1): 41–9. doi:10.1042/BJ20021963. PMC 1223573. PMID 12747803.
- González B, Schell MJ, Letcher AJ, Veprintsev DB, Irvine RF, Williams RL (September 2004). "Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase". Molecular Cell. 15 (5): 689–701. doi:10.1016/j.molcel.2004.08.004. PMID 15350214.
- Kato H, Uzawa K, Onda T, Kato Y, Saito K, Nakashima D, Ogawara K, Bukawa H, Yokoe H, Tanzawa H (April 2006). "Down-regulation of 1D-myo-inositol 1,4,5-trisphosphate 3-kinase A protein expression in oral squamous cell carcinoma". International Journal of Oncology. 28 (4): 873–81. doi:10.3892/ijo.28.4.873. PMID 16525636.