| Envelope glycoprotein gp120 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | GP120 | ||||||||
| Pfam | PF00516 | ||||||||
| InterPro | IPR000777 | ||||||||
| SCOP2 | 1gc1 / SCOPe / SUPFAM | ||||||||
| |||||||||
Envelope glycoprotein GP120 (or gp120) is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1984.[1] The 120 in its name comes from its molecular weight of 120 kDa. Gp120 is essential for virus entry into cells as it plays a vital role in attachment to specific cell surface receptors. These receptors are DC-SIGN,[2] Heparan Sulfate Proteoglycan[3] and a specific interaction with the CD4 receptor,[4] particularly on helper T-cells. Binding to CD4 induces the start of a cascade of conformational changes in gp120 and gp41 that lead to the fusion of the viral membrane with the host cell membrane. Binding to CD4 is mainly electrostatic although there are van der Waals interactions and hydrogen bonds.[5]
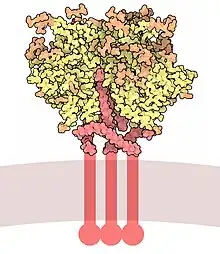
Gp120 is coded by the HIV env gene, which is around 2.5 kb long and codes for around 850 amino acids.[6] The primary env product is the protein gp160, which gets cleaved to gp120 (~480 amino acids) and gp41 (~345 amino acids) in the endoplasmatic reticulum by the cellular protease furin.[7] The crystal structure of core gp120 shows an organization with an outer domain, an inner domain with respect to its termini and a bridging sheet. Gp120 is anchored to the viral membrane, or envelope, via non-covalent bonds with the transmembrane glycoprotein, gp41. Three gp120s and gp41s combine in a trimer of heterodimers to form the envelope spike,[8] which mediates attachment to and entry into the host cell.
Variability
Since gp120 plays a vital role in the ability of HIV-1 to enter CD4+ cells, its evolution is of particular interest. Many neutralizing antibodies bind to sites located in variable regions of gp120, so mutations in these regions will be selected for strongly.[9] The diversity of env has been shown to increase by 1-2% per year in HIV-1 group M and the variable units are notable for rapid changes in amino acid sequence length. Increases in gp120 variability result in significantly elevated levels of viral replication, indicating an increase in viral fitness in individuals infected by diverse HIV-1 variants.[10] Further studies have shown that variability in potential N-linked glycosylation sites (PNGSs) also result in increased viral fitness. PNGSs allow for the binding of long-chain carbohydrates to the high variability regions of gp120, so the authors hypothesize that the number of PNGSs in env might affect the fitness of the virus by providing more or less sensitivity to neutralizing antibodies. The presence of large carbohydrate chains extending from gp120 might obscure possible antibody binding sites.[11]
The boundaries of the potential to add and eliminate PNGSs are naively explored by growing viral populations following each new infection.[12] While the transmitting host has developed a neutralizing antibody response to gp120, the newly infected host lacks immune recognition of the virus. Sequence data shows that initial viral variants in an immunologically naïve host have few glycosylation sites and shorter exposed variable loops. This may facilitate viral ability to bind host cell receptors.[13] As the host immune system develops antibodies against gp120, immune pressures seem to select for increased glycosylation, particularly on the exposed variable loops of gp120.[14] Consequently, insertions in env, which confer more PNGSs on gp120 may be more tolerated by the virus as higher glycan density promotes the viral ability to evade antibodies and thus promotes higher viral fitness.[15] In considering how much PNGS density could theoretically change, there may be an upper bound to PNGS number due to its inhibition of gp120 folding, but if the PNGS number decreases substantially, then the virus is too easily detected by neutralizing antibodies.[12] Therefore, a stabilizing selection balance between low and high glycan densities is likely established. A lower number of bulky glycans improves viral replication efficiency and higher number on the exposed loops aids host immune evasion via disguise.
The relationship between gp120 and neutralizing antibodies is an example of Red Queen evolutionary dynamics. Continuing evolutionary adaptation is required for the viral envelope protein to maintain fitness relative to the continuing evolutionary adaptations of the host immune neutralizing antibodies, and vice versa, forming a coevolving system.[15]
Vaccine target
Since CD4 receptor binding is the most obvious step in HIV infection, gp120 was among the first targets of HIV vaccine research. Efforts to develop HIV vaccines targeting gp120, however, have been hampered by the chemical and structural properties of gp120, which make it difficult for antibodies to bind to it. gp120 can also easily be shed from the surface of the virus and captured by T cells due to its loose binding with gp41. A conserved region in the gp120 glycoprotein that is involved in the metastable attachment of gp120 to CD4 has been identified and targeting of invariant region has been achieved with a broadly neutralising antibody, IgG1-b12.[16] [17]
NIH research published in Science reports the isolation of 3 antibodies that neutralize 90% of HIV-1 strains at the CD4bs region of gp120, potentially offering a therapeutic and vaccine strategy. However, most antibodies that bind the CDbs region of gp120 do not neutralize HIV,[18] and rare ones that do such as IgG1-b12 have unusual properties such as asymmetry of the Fab arms[19] or in their positioning.[20] Unless a gp120-based vaccine can be designed to elicit antibodies with strongly neutralizing antiviral properties, there is concern that breakthrough infection leading to humoral production of high levels of non-neutralizing antibodies targeting the CD4 binding site of gp120 is associated with faster disease progression to AIDS.[21]
Competition
The protein gp120 is necessary during the initial binding of HIV to its target cell. Consequently, anything which binds to gp120 or its targets can physically block gp120 from binding to a cell. Only one such agent, Maraviroc, which binds the co-receptor CCR5 is currently licensed and in clinical use. No agent targeting gp120's main first cellular interaction partner, CD4, is currently licensed since interfering with such a central molecule of the immune system can cause toxic side effects, such as the anti-CD4 monoclonal antibody OKT4. Targeting gp120 itself has proven extremely difficult due to its high degree of variability and shielding. Fostemsavir (BMS-663068) is a methyl phosphate prodrug of the small molecule inhibitor BMS-626529, which prevents viral entry by binding to the viral envelope gp120 and interfering with virus attachment to the host CD4 receptor.[22]
HIV dementia
The HIV viral protein gp120 induces apoptosis of neuronal cells by inhibiting levels of furin and tissue plasminogen activator, enzymes responsible for converting pBDNF to mBDNF.[23] gp120 induces mitochondrial-death proteins like caspases which may influence the upregulation of the death receptor Fas leading to apoptosis of neuronal cells,[24] gp120 induces oxidative stress in the neuronal cells,[25] and it is also known to activate STAT1 and induce interleukins IL-6 and IL-8 secretion in neuronal cells.[26]
See also
References
- ↑ Sodroski, Joseph; Patarca, Roberto; Perkins, Dennis; Briggs, Debra; Tun-Hou, Lee; Essex, Myron; Coligan, John; Wong-Staal, Flossie; Gallo, Robert C. (1984). "Sequence of the Envelope Glycoprotein Gene of Type II Human T Lymphotropic Virus". Science. 225 (4660): 421–424. Bibcode:1984Sci...225..421S. doi:10.1126/science.6204380. PMID 6204380.
- ↑ Curtis BM, Scharnowske S, Watson AJ (September 1992). "Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120". Proc. Natl. Acad. Sci. U.S.A. 89 (17): 8356–60. Bibcode:1992PNAS...89.8356C. doi:10.1073/pnas.89.17.8356. PMC 49917. PMID 1518869.
- ↑ de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P (December 2007). "Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1". Proc. Natl. Acad. Sci. U.S.A. 104 (49): 19464–9. Bibcode:2007PNAS..10419464D. doi:10.1073/pnas.0703747104. PMC 2148312. PMID 18040049.
- ↑ Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA (1984). "The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus". Nature. 312 (5996): 763–7. Bibcode:1984Natur.312..763D. doi:10.1038/312763a0. PMID 6096719. S2CID 4349809.
- ↑ Korkut, A; Hendrickson, WA (2012). "Structural Plasticity and Conformational Transitions of HIV Envelope Glycoprotein gp120". PLOS ONE. 7 (12): e52170. Bibcode:2012PLoSO...752170K. doi:10.1371/journal.pone.0052170. PMC 3531394. PMID 23300605.
- ↑ Kuiken, C., Leitner, T., Foley, B., et al. (2008). "HIV Sequence Compendium", Los Alamos National Laboratory.
- ↑ Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk HD, Garten W (November 1992). "Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160". Nature. 360 (6402): 358–61. Bibcode:1992Natur.360..358H. doi:10.1038/360358a0. PMID 1360148. S2CID 4306605.
- ↑ Zhu P, Winkler H, Chertova E, Taylor KA, Roux KH (November 2008). "Cryoelectron tomography of HIV-1 envelope spikes: further evidence for tripod-like legs". PLOS Pathog. 4 (11): e1000203. doi:10.1371/journal.ppat.1000203. PMC 2577619. PMID 19008954.
- ↑ Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG (1998). "The antigenic structure of the HIV gp120 envelope glycoprotein". Nature. 393 (6686): 705–711. Bibcode:1998Natur.393..705W. doi:10.1038/31514. PMID 9641684. S2CID 4384110.
- ↑ Novitsky V, Lagakos S, Herzig M, Bonney C, Kebaabetswe L, Rossenkhan R, Nkwe D, Margolin L, Musonda R, Moyo S, Woldegabriel E, van Widenfelt E, Makhema J, Essex M (January 2009). "Evolution of proviral gp120 over the first year of HIV-1 subtype C infection". Virology. 383 (1): 47–59. doi:10.1016/j.virol.2008.09.017. PMC 2642736. PMID 18973914.
- ↑ Wood N, Bhattacharya T, Keele BF, Giorgi E, Liu M, Gaschen B, Daniels M, Ferrari G, Haynes BF, McMichael A, Shaw GM, Hahn BH, Korber B, Seoighe C (May 2009). "HIV evolution in early infection: selection pressures, patterns of insertion and deletion, and the impact of APOBEC". PLOS Pathog. 5 (5): e1000414. doi:10.1371/journal.ppat.1000414. PMC 2671846. PMID 19424423.
- 1 2 Korber, Bette; Kuiken, Carla; Haigwood, Nancy; Foley, Brian; Blay, Wendy; Gaschen, Brian; Zhang, Ming (2004-12-01). "Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin". Glycobiology. 14 (12): 1229–1246. doi:10.1093/glycob/cwh106. ISSN 0959-6658. PMID 15175256.
- ↑ Liu Y, Curlin ME, Diem K, Zhao H, Ghosh AK, Zhu H, Woodward AS, Maenza J, Stevens CE, Stekler J, Collier AC, Genowati I, Deng WZioni R, Corey L, Zhu T, Mullins JI (May 2008). "Env length and N-linked glycosylation following transmission of human immunodeficiency virus Type 1 subtype B viruses". Virology. 374 (2): 229–33. doi:10.1016/j.virol.2008.01.029. PMC 2441482. PMID 18314154.
- ↑ Pantophlet R, Burton DR (2006). "GP120: target for neutralizing HIV-1 antibodies". Annu. Rev. Immunol. 24: 739–69. doi:10.1146/annurev.immunol.24.021605.090557. PMID 16551265.
- 1 2 Frost SD, Wrin T, Smith DM, Kosakovsky Pond SL, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropoulos CJ, Little SJ, Richman DD (December 2005). "Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection". Proc. Natl. Acad. Sci. U.S.A. 102 (51): 18514–9. Bibcode:2005PNAS..10218514F. doi:10.1073/pnas.0504658102. PMC 1310509. PMID 16339909.
- ↑ Barbas CF, Björling E, Chiodi F, Dunlop N, Cababa D, Jones TM, Zebedee SL, Persson MA, Nara PL, Norrby E (October 1992). "Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro". Proc. Natl. Acad. Sci. U.S.A. 89 (19): 9339–43. Bibcode:1992PNAS...89.9339B. doi:10.1073/pnas.89.19.9339. PMC 50122. PMID 1384050.
- ↑ Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, Burton DR, Dimitrov DS, Sodroski J, Wyatt R, Nabel GJ, Kwong PD (2007). "Structural definition of a conserved neutralization epitope on HIV-1 gp120". Nature. 445 (7129): 732–737. Bibcode:2007Natur.445..732Z. doi:10.1038/nature05580. PMC 2584968. PMID 17301785.
- ↑ Pantophlet, Ralph; Ollmann Saphire, Erica; Poignard, Pascal; Parren, Paul W. H. I.; Wilson, Ian A.; Burton, Dennis R. (2003-01-01). "Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120". Journal of Virology. 77 (1): 642–658. doi:10.1128/jvi.77.1.642-658.2003. ISSN 0022-538X. PMC 140633. PMID 12477867.
- ↑ Ashish, null; Solanki, Ashish K.; Boone, Christopher D.; Krueger, Joanna K. (2010-01-01). "Global structure of HIV-1 neutralizing antibody IgG1 b12 is asymmetric". Biochemical and Biophysical Research Communications. 391 (1): 947–951. doi:10.1016/j.bbrc.2009.11.170. ISSN 1090-2104. PMID 19995532.
- ↑ Solanki, Ashish K.; Rathore, Yogendra S.; Badmalia, Maulik D.; Dhoke, Reema R.; Nath, Samir K.; Nihalani, Deepak; Ashish (2014-12-12). "Global Shape and Ligand Binding Efficiency of the HIV-1-neutralizing Antibodies Differ from Those of Antibodies That Cannot Neutralize HIV-1". The Journal of Biological Chemistry. 289 (50): 34780–34800. doi:10.1074/jbc.M114.563486. ISSN 0021-9258. PMC 4263879. PMID 25331945.
- ↑ Chien, Peter C.; Cohen, Sandra; Kleeberger, Cynthia; Giorgi, Janis; Phair, John; Zolla-Pazner, Susan; Hioe, Catarina E. (2002-07-15). "High levels of antibodies to the CD4 binding domain of human immunodeficiency virus type 1 glycoprotein 120 are associated with faster disease progression". The Journal of Infectious Diseases. 186 (2): 205–213. doi:10.1086/341297. ISSN 0022-1899. PMID 12134256.
- ↑ aidsinfo.nih.gov/drugs/508/bms-663068/0/professional
- ↑ Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I (2012). "Human Immunodeficiency Virus Type 1 Alters Brain-Derived Neurotrophic Factor Processing in Neurons". The Journal of Neuroscience. 32 (28): 9477–9484. doi:10.1523/JNEUROSCI.0865-12.2012. PMC 3408006. PMID 22787033.
- ↑ Thomas S, Mayer L, Sperber K (2009). "Mitochondria influence Fas expression in gp120-induced apoptosis of neuronal cells". Int. J. Neurosci. 119 (2): 157–65. doi:10.1080/00207450802335537. PMID 19125371. S2CID 25456692.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Price TO, Ercal N, Nakaoke R, Banks WA (May 2005). "HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells". Brain Res. 1045 (1–2): 57–63. doi:10.1016/j.brainres.2005.03.031. PMID 15910762. S2CID 7362454.
- ↑ Yang B, Akhter S, Chaudhuri A, Kanmogne GD (March 2009). "HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood–brain barrier: modulatory effects of STAT1 signaling". Microvasc. Res. 77 (2): 212–9. doi:10.1016/j.mvr.2008.11.003. PMC 3715090. PMID 19103208.
Further reading
- Human Immunodeficiency Virus Glycoprotein 120
- Vashistha H, Husain M, Kumar D, Singhal PC (2009). "Tubular cell HIV-1 gp120 expression induces caspase 8 activation and apoptosis". Ren Fail. 31 (4): 303–12. doi:10.1080/08860220902780101. PMID 19462280. S2CID 205593494.
External links
- https://web.archive.org/web/20060219135317/http://www.aidsmap.com/en/docs/4406022B-85D7-4A9B-B700-91336CBB6B18.asp
- http://www.mcld.co.uk/hiv/?q=gp120 Archived 2008-06-24 at the Wayback Machine
- http://www.ebi.ac.uk/interpro/IEntry?ac=IPR000777
- Vashistha, H.; Husain, M.; Kumar, D.; Singhal, P. C. (2009). "Tubular Cell HIV-1 gp120 Expression Induces Caspase 8 Activation and Apoptosis". Renal Failure. 31 (4): 303–312. doi:10.1080/08860220902780101. PMID 19462280. S2CID 205593494.