 | |
| Names | |
|---|---|
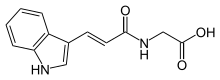
| Preferred IUPAC name
[(2E)-3-(1H-Indol-3-yl)prop-2-enamido]acetic acid | |
| Other names
indoleacrylic glycine | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| Properties | |
| C13H12N2O3 | |
| Molar mass | 244.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Indolyl-3-acryloylglycine, also known as trans-indolyl-3-acryloylglycine, or IAG for short, is a compound consisting of an indole group attached to an acrylic acid moiety, which is in turn attached to a glycine molecule. This compound has been shown to isomerize when exposed to light.[1] It is likely a metabolic intermediate in the biosynthesis of tryptophan,[2] and is synthesized from tryptophan via indolepropionic acid and indoleacrylicacid (IAcrA). It is also likely that IAcrA is converted into IAG in the gut wall.[3] It may also be produced by certain elements of the mammalian gut microbiota by phenylalanine ammonia-lyase.[4] Identifiable in the urine by high-performance liquid chromatography, it may be a biomarker for autism spectrum disorders, as demonstrated by the research of Paul Shattock[5][6][7] and other researchers from Australia.[8] These researchers have reported that urinary levels of IAG are much higher in autistic children than in controls; however, other researchers have found no association between IAG concentrations in the urine and autism.[9] Its excretion in the urine may also be changed in Hartnup disease and celiac disease,[10] as well as photodermatosis, muscular dystrophy, and liver cirrhosis.[11]
References
- ↑ Mills, M. J.; Savery, D.; Shattock, P. E. (1998). "Rapid analysis of low levels of indolyl-3-acryloylglycine in human urine by high-performance liquid chromatography". Journal of Chromatography B. 712 (1–2): 51–58. doi:10.1016/S0378-4347(98)00153-4. PMID 9698228.
- ↑ Marklová, E. (1999). "Where does indolylacrylic acid come from". Amino Acids. 17 (4): 401–413. doi:10.1007/BF01361665. PMID 10707769.
- ↑ Shattock, Paul. "The Role of Tryptophan in Autism and Related Disorders" (PDF). The Nutrition Practitioner (Summer 2006).
- ↑ Clayton, T. A. (2012). "Metabolic differences underlying two distinct rat urinary phenotypes, a suggested role for gut microbial metabolism of phenylalanine and a possible connection to autism". FEBS Letters. 586 (7): 956–961. doi:10.1016/j.febslet.2012.01.049. PMID 22306194.
- ↑ Anderson, R. J.; Bendell, D. J.; Garnett, I.; Groundwater, P. W.; Lough, W. J.; Mills, M. J.; Savery, D.; Shattock, P. E. G. (2002). "Identification of indolyl-3-acryloylglycine in the urine of people with autism". Journal of Pharmacy and Pharmacology. 54 (2): 295–298. doi:10.1211/0022357021778349. PMID 11858215.
- ↑ Bull, G.; Shattock, P.; Whiteley, P.; Anderson, R.; Groundwater, P. W.; Lough, J. W.; Lees, G. (2003). "Indolyl-3-acryloylglycine (IAG) is a putative diagnostic urinary marker for autism spectrum disorders". Medical Science Monitor. 9 (10): CR422–CR425. PMID 14523330.
- ↑ Whiteley, P.; Mrpharms, P. S. (2003). "What Makes Trans‐indolyl‐3‐acryloylglycine Identified by High‐performance Liquid Chromatography Relevant to Pervasive Developmental Disorders?". Journal of Nutritional and Environmental Medicine. 13 (4): 231. doi:10.1080/13590840310001641996.
- ↑ Wang, L.; Angley, M. T.; Gerber, J. P.; Young, R. L.; Abarno, D. V.; McKinnon, R. A.; Sorich, M. J. (2009). "Is urinary indolyl-3-acryloylglycine a biomarker for autism with gastrointestinal symptoms?". Biomarkers. 14 (8): 596–603. doi:10.3109/13547500903183962. PMID 19697973.
- ↑ Wright, B.; Brzozowski, A. M.; Calvert, E.; Farnworth, H.; Goodall, D. M.; Holbrook, I.; Imrie, G.; Jordan, J.; Kelly, A.; Miles, J.; Smith, R.; Town, J. (2005). "Is the presence of urinary indolyl-3-acryloylglycine associated with autism spectrum disorder?". Developmental Medicine & Child Neurology. 47 (3): 190–192. doi:10.1017/S0012162205000344. PMID 15739724.
- ↑ Keszthelyi, D.; Troost, F. J.; Masclee, A. A. M. (2009). "Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function". Neurogastroenterology & Motility. 21 (12): 1239–1249. doi:10.1111/j.1365-2982.2009.01370.x. PMID 19650771.
- ↑ Marklová, E.; Fojtásková, A. (1996). "High-performance liquid chromatographic profiling of indolylacryloylglycine and its possible precursors in body fluids". Journal of Chromatography A. 730 (1–2): 133–137. doi:10.1016/0021-9673(95)00943-4. PMID 8680585.