 | |
 | |
| Names | |
|---|---|
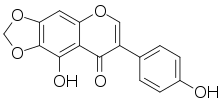
| IUPAC name
4′,9-Dihydroxy-6,7-[methylenebis(oxy)]isoflavone | |
| Systematic IUPAC name
9-Hydroxy-7-(4-hydroxyphenyl)-2H,8H-[1,3]dioxolo[4,5-g][1]benzopyran-8-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H10O6 | |
| Molar mass | 298.24 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Irilone is an isoflavone, a type of flavonoid. It can be found in Trifolium pratense (red clover),[1] in Iris unguicularis[2] and in Iris germanica.[3]
References
- ↑ The red clover isoflavone irilone is largely resistant to degradation by the human gut microbiota. Annett Braune, Ronald Maul, Nils Helge Schebb, Sabine E. Kulling and Michael Blaut, Molecular Nutrition & Food Research, 8 Dec 2009
- ↑ New and Known Constituents from Iris unguicularis and Their Antioxidant Activity. Atta-ur-Rahman, Sumaira Hareem, M. Iqbal Choudhary, Bilge Sener, Ahmed Abbaskhan, Hina Siddiqui, Shazia Anjum, Ilkay Orhan, Ilhan Gurbuz and Filiz Ayanoglu, HeteroCycles, 2010, Special issue, Vol 82, No. 1, pages 813–824, doi:10.3987/COM-10-S(E)6
- ↑ Lipase-catalyzed regioselective protection/deprotection of hydroxyl groups of the isoflavone irilone isolated from Iris germanica. Nighat Nazir, Surrinder Koul, Mushtaq Ahmad Qurishi, Subhash Chandra Taneja and Ghulam Nabi Qazi, Biocatalysis and Biotransformation, 2 December 2008, 1029–2446, Volume 27, Issue 2, Pages 118–123, INIST 21235726
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.