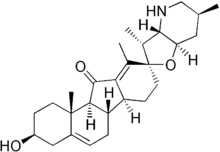
 | |
| Names | |
|---|---|
| IUPAC name
3β-Hydroxy-17β,23β-epoxyveratraman-11-one | |
| Systematic IUPAC name
(2′R,3S,3′R,3′aS,6′S,6aS,6bS,7′aR,11aS,11bR)-3-Hydroxy-3′,6′,10,11b-tetramethyl-2,3,3′a,4,4′,5′,6,6′,6a,6b,7,7′,7′a,8,11a,11b-hexadecahydro-3′H-spiro[benzo[a]fluorene-9,2′-furo[3,2-b]pyridin]-11(1H)-one | |
| Other names
(3β,23β)-17,23-Epoxy-3-hydroxyveratraman-11-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.745 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H39NO3 | |
| Molar mass | 425.60 g/mol |
| Solubility | 10 mg/mL in EtOH 6 mg/mL in DMF |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Jervine is a steroidal alkaloid with molecular formula C27H39NO3 which is derived from the plant genus Veratrum. Similar to cyclopamine, which also occurs in the genus Veratrum, it is a teratogen implicated in birth defects when consumed by animals during a certain period of their gestation.
Physiological effects
Jervine is a potent teratogen causing birth defects in vertebrates. In severe cases it can cause cyclopia and holoprosencephaly.
Mechanism of action
Jervine's biological activity is mediated via its interaction with the 7 pass trans membrane protein smoothened. Jervine binds with and inhibits smoothened, which is an integral part of the hedgehog signaling pathways.[1] With smoothened inhibited, the GLI1 transcription cannot be activated and hedgehog target genes cannot be transcribed.
References
- ↑ Chen, J; Taipale, J; Cooper, M. (2002). "Inhibition of Hedgehog Signaling by direct binding of Cyclopamine to Smoothened". Genes Dev. 16 (21): 2743–2748. doi:10.1101/gad.1025302. PMC 187469. PMID 12414725.