 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
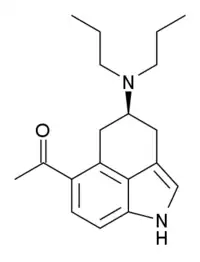
| Formula | C19H26N2O |
| Molar mass | 298.430 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
LY-293284 is a research chemical developed by the pharmaceutical company Eli Lilly and used for scientific studies. It acts as a potent and selective 5-HT1A receptor full agonist. It was derived through structural simplification of the ergoline based psychedelic LSD,[1] but is far more selective for 5-HT1A with over 1000x selectivity over other serotonin receptor subtypes and other targets.[2] It has anxiogenic effects in animal studies.[3]
See also
References
- ↑ Monte AP, Marona-Lewicka D, Lewis MM, Mailman RB, Wainscott DB, Nelson DL, Nichols DE (June 1998). "Substituted naphthofurans as hallucinogenic phenethylamine-ergoline hybrid molecules with unexpected muscarinic antagonist activity". Journal of Medicinal Chemistry. 41 (12): 2134–45. doi:10.1021/jm980076u. PMID 9622555.
- ↑ Foreman MM, Fuller RW, Rasmussen K, Nelson DL, Calligaro DO, Zhang L, Barrett JE, Booher RN, Paget CJ, Flaugh ME (September 1994). "Pharmacological characterization of LY293284: A 5-HT1A receptor agonist with high potency and selectivity". The Journal of Pharmacology and Experimental Therapeutics. 270 (3): 1270–81. PMID 7523657.
- ↑ Cao BJ, Rodgers RJ (October 1998). "Comparative effects of novel 5-HT1A receptor ligands, LY293284, LY315712 and LY297996, on plus-maze anxiety in mice". Psychopharmacology. 139 (3): 185–94. doi:10.1007/s002130050703. PMID 9784072. S2CID 9466299.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.