 | |
| Names | |
|---|---|
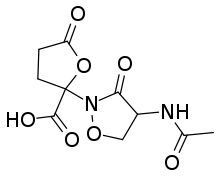
| IUPAC name
2-(4-Acetamido-3-oxo-1,2-oxazolidin-2-yl)-5-oxooxolane-2-carboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12N2O7 | |
| Molar mass | 272.213 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lactivicin is a non-beta-lactam antibiotic that is active against a range of Gram-positive and Gram-negative bacteria.[1] Lactivicin demonstrates a similar affinity for penicillin-binding proteins to beta-lactam antibiotics and is also susceptible to beta-lactamase enzymes.[2]
References
- ↑ Nozaki Y, Katayama N, Harada S, Ono H, Okazaki H (1989). "Lactivicin, a naturally occurring non-beta-lactam antibiotic having beta-lactam-like action: biological activities and mode of action". J. Antibiot. 42 (10): 84–93. doi:10.7164/antibiotics.42.84. PMID 2493440.
- ↑ Harada S, Tsubotani S, Hida T, Ono H, Okazaki H (1986). "Structure of lactivicin, an antibiotic having a new nucleus and similar biological activities to β-lactam antibiotics". Tetrahedron Letters. 27 (51): 6229–6232. doi:10.1016/S0040-4039(00)85439-8.
External links
- "Mechanism Of Unusual Antibiotic". Chemical & Engineering News. 85 (33): 13. August 13, 2007.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.