 | |
| Names | |
|---|---|
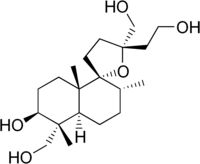
| IUPAC name
(13S)-9α,13-Epoxy-8α-labdane-3β,15,16,18-tetrol | |
| Systematic IUPAC name
(1R,2R,4aS,5R,5′S,6S)-5′-(2-Hydroxyethyl)-5,5′-bis(hydroxymethyl)-2,5,8a-trimethyloctahydro-2H-spiro[naphthalene-1,2′-oxolan]-6-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H36O5 | |
| Molar mass | 356.495 g/mol |
| Appearance | Light grey crystalline solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lagochilin is a bitter diterpene that forms a grey crystalline solid. It is found in various plants from the genus Lagochilus, most notably Lagochilus inebrians, and is thought to be responsible for the sedative, hypotensive and hemostatic effects of this plant.[1][2][3][4]
References
- ↑ Pulatova TP, Khazanovich RL (1962). "[On the alkaloid content of some Lagochilus species and on the nature of lagochiline]". Aptechnoe Delo. 6: 29–32. PMID 13972488.
- ↑ Mavlyankulova ZI, Zainutdinov UN, Mukhamedkhanov SI, Leont'ev VD, Aslanov KA (1980). "Investigation of the Lagochiline Acetylation Reaction". Khimiya Prirodnykh Soedinenii. 1: 46–9.
- ↑ Chizhov OS, Kessenikh AV, Yakovlev IP, Zolotarev BM, Petukhov VA, Zelinsky ND (January 1969). "Structure of lagochilin". Tetrahedron Letters. 10 (17): 1361–1364. doi:10.1016/S0040-4039(01)87886-2.
- ↑ Chizhov OS, Kessenikh AV, Yakovlev IP, Zolotarev BM, Petukhov VA (1970). "The structure of lagochilin". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science. 19 (9): 1866–1972. doi:10.1007/BF00849762.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.