 | |
| Names | |
|---|---|
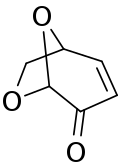
| Preferred IUPAC name
(1S,5R)-6,8-Dioxabicyclo[3.2.1]oct-2-en-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.111 g·mol−1 |
| Appearance | yellow liquid |
| Density | 1.304 g/cm3 (20 °C) [1] |
| Boiling point | 231 °C; 448 °F; 504 K[1] |
| Vapor pressure | 6.2 Pa (25 °C) [1] |
Refractive index (nD) |
1.5065 (20 °C)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Levoglucosenone is an organic compound with the formula [OCH2(CH)4CO2]. A pale yellow liquid, it is an unsaturated bicyclic ketone-diether formed from levoglucosan by loss of two molecules of water. As a product of the acid-catalysed pyrolysis of cellulose, D-glucose, and levoglucosan, this liquid hydrocarbon is of interest as a biofuel and biofeedstock.[2]
Production
The compound was first identified in 1970 as a product of the thermal decomposition of cellulose.[3]
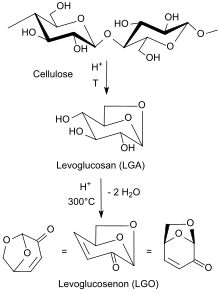
The primary way of obtaining levoglucosenone is via pyrolysis of carbohydrates, particularly cellulose. Levoglucosenone can be derived from biomass or from other cellulosic materials including domestic/commercial waste paper. The availability of multiple sources is a key advantage when compared to other platform chemicals which are solely derived from biomass.
The title compound is produced when cellulose is heated above 170 °C with sulfuric acid with various additives.[2] Alongside levoglucosenone as a major product, 2-furfuraldehyde is sometimes formed in 5-10%. The bio-oil can be vacuum distilled, resulting in purified levoglucosenone.[4] The use of polar, aprotic solvents such as THF, γ-valerolactone and sulfolane has been found to improve pyrolytic yields, as the solvents cause swelling of the cellulose and inhibit repolymerisation back to levoglucosan. These solvents also promote catalytic dehydration of levoglucosan to levoglucosenone.[5]
Microwave irradiation of microcrystalline cellulose can also be used to produce levoglucosenone.[6]

Cellulose-containing waste from biorefineries can also be converted into 6-8% LGO under microwave irradiation in addition to the usual decomposition products such as hydroxymethylfurfural HMF, formic acid, formaldehyde, CO2 and water.[7]
Reactions
As a highly functionalized, chiral compound, levoglucosenone is a precursor to a variety of compounds.[8] Levoglucosenone is a promising bio-renewable platform for the production of commodity chemicals, being especially interesting the new insight provided by Huber and co-workers into how to transform this molecule into α,ω-diols, monomers for the production of polyesters and polyurethanes.[9]
Palladium on carbon (and related Pd- and Pt-based catalysts) act on the title compound by hydrogenation (i.e. dihydrolevoglucosenone and levoglucosanol) and hydrogenolysis (i.e. tetrahydrofurandimethanol (THFDM) and 1,6-hexanediol). [10][11]
Levoglucosenone is a Michael acceptor enabling its conversion to a variety of derivatives.[12]
See also
References
- 1 2 3 4 Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics. 40 (11): 102. Bibcode:2019IJT....40..102B. doi:10.1007/s10765-019-2570-9.
- 1 2 Klepp, J.; Dillon, W.; Lin, Y.; Feng, P.; Greatrex, B. W. (2020). "Preparation of (-)-Levoglucosenone from Cellulose Using Sulfuric Acid in Polyethylene Glycol". Organic Syntheses. 97: 38–53. doi:10.15227/orgsyn.097.0038. S2CID 216214489.
- ↑ Tsuchiya, Yoshio; Sumi, Kikuo (1970). "Thermal decomposition products of cellulose". Journal of Applied Polymer Science. 14 (8): 2003–2013. doi:10.1002/app.1970.070140808. ISSN 0021-8995. S2CID 98167855.
- ↑ Comba, María B.; Tsai, Yi-hsuan; Sarotti, Ariel M.; Mangione, María I.; Suárez, Alejandra G.; Spanevello, Rolando A. (2017-12-19). "Levoglucosenone and Its New Applications: Valorization of Cellulose Residues". European Journal of Organic Chemistry. 2018 (5): 590–604. doi:10.1002/ejoc.201701227. ISSN 1434-193X.
- ↑ Kudo, Shinji; Goto, Nozomi; Sperry, Jonathan; Norinaga, Koyo; Hayashi, Jun-ichiro (2016-12-21). "Production of Levoglucosenone and Dihydrolevoglucosenone by Catalytic Reforming of Volatiles from Cellulose Pyrolysis Using Supported Ionic Liquid Phase". ACS Sustainable Chemistry & Engineering. 5 (1): 1132–1140. doi:10.1021/acssuschemeng.6b02463. ISSN 2168-0485.
- ↑ Sarotti, Ariel M.; Spanevello, Rolando A.; Suárez, Alejandra G. (2007). "An efficient microwave-assisted green transformation of cellulose into levoglucosenone. Advantages of the use of an experimental design approach". Green Chemistry. 9 (10): 1137. doi:10.1039/b703690f. ISSN 1463-9262.
- ↑ Clark, J. H.; McQueen-Mason, S. J.; Raverty, W. D.; Farmer, T. J.; Simister, R.; Gomez, L. D.; Macquarrie, D. J.; Budarin, V. L.; Fan, J. (2016-08-02). "A new perspective in bio-refining: levoglucosenone and cleaner lignin from waste biorefinery hydrolysis lignin by selective conversion of residual saccharides". Energy & Environmental Science. 9 (8): 2571–2574. doi:10.1039/C6EE01352J. ISSN 1754-5706.
- ↑ Sarotti, Ariel M.; Zanardi, María M.; Spanevello, Rolando A.; Suárez, Alejandra G. (2012-11-01). "Recent applications of levoglucosenone as chiral synthon". Current Organic Synthesis. 9 (4): 439–459. doi:10.2174/157017912802651401.
- ↑ Huber, George W.; Dumesic, James A.; Hermans, Ive; Maravelias, Christos T.; Banholzer, Williams F.; Walker, Theodore; Burt, Samuel P.; Brentzel, Zachary J.; Alonso, David M. (2017-09-20). "New catalytic strategies for α,ω-diols production from lignocellulosic biomass". Faraday Discussions. 202: 247–267. Bibcode:2017FaDi..202..247H. doi:10.1039/C7FD00036G. ISSN 1364-5498. PMID 28678237.
- ↑ Krishna, Siddarth H.; McClelland, Daniel J.; Rashke, Quinn A.; Dumesic, James A.; Huber, George W. (2016-12-12). "Hydrogenation of levoglucosenone to renewable chemicals". Green Chemistry. 19 (5): 1278–1285. doi:10.1039/C6GC03028A. OSTI 1477850.
- ↑ Mazarío, Jaime; Parreño Romero, Míriam; Concepción, Patricia; Chávez-Sifontes, Marvin; Spanevello, Rolando A.; Comba, María B.; Suárez, Alejandra G.; Domine, Marcelo E. (2019-07-26). "Tuning zirconia-supported metal catalysts for selective one-step hydrogenation of levoglucosenone". Green Chemistry. 21 (17): 4769–4785. doi:10.1039/C9GC01857C. hdl:11336/108039. ISSN 1463-9270. S2CID 199647263.
- ↑ Lefebvre, Corentin; Van Gysel, Terence; Michelin, Clément; Rousset, Elodie; Djiré, Djibril; Allais, Florent; Hoffmann, Norbert (2022). "Photocatalytic Radical Addition to Levoglucosenone" (PDF). European Journal of Organic Chemistry. 2022. doi:10.1002/ejoc.202101298. S2CID 245305312.