 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.206.772 |
| Chemical and physical data | |
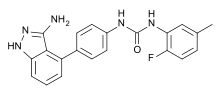
| Formula | C17H15FN5O |
| Molar mass | 324.339 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Linifanib (ABT-869) is a structurally novel, potent inhibitor of receptor tyrosine kinases (RTK), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) with IC50 of 0.2, 2, 4, and 7 nM for human endothelial cells, PDGF receptor beta (PDGFR-β), KDR, and colony stimulating factor 1 receptor (CSF-1R), respectively. It has much less activity (IC50s > 1 μM) against unrelated RTKs, soluble tyrosine kinases, or serine/threonine kinases. In vivo linifanib is effective orally in mechanism-based murine models of VEGF-induced uterine edema (ED50 = 0.5 mg/kg) and corneal angiogenesis (>50%inhibition, 15 mg/kg).[1][2]
References
- ↑ Albert DH, Tapang P, Magoc TJ, Pease LJ, Reuter DR, Wei RQ, et al. (April 2006). "Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor". Molecular Cancer Therapeutics. 5 (4): 995–1006. doi:10.1158/1535-7163.MCT-05-0410. PMID 16648571.
- ↑ Guo J, Marcotte PA, McCall JO, Dai Y, Pease LJ, Michaelides MR, et al. (April 2006). "Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors". Molecular Cancer Therapeutics. 5 (4): 1007–13. doi:10.1158/1535-7163.MCT-05-0359. PMID 16648572.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.