This is a list of aminorex analogues. Aminorex itself is a stimulant drug with a 5-phenyl-2-amino-oxazoline structure. It was developed in the 1960s as an anorectic,[1][2][3] but withdrawn from sale after it was discovered that extended use produced pulmonary hypertension, often followed by heart failure, which resulted in a number of deaths.[4] A designer drug analogue 4-methylaminorex appeared on the illicit market in the late 1980s but did not attract significant popularity due to its steep dose-response curve and tendency to produce seizures.[5][6][7][8] Pemoline, the 4-keto derivative of aminorex, had been discovered several years earlier,[9] and derivatives of this type appeared to be effective stimulants with comparatively low toxicity.[10][11] Pemoline was sold for around 25 years as a therapy for ADHD and relief of fatigue, before being withdrawn from the market in 2005 because of rare but serious cases of liver failure.[12][13][14][15] More recently in around 2014 another derivative 4,4'-dimethylaminorex started to be sold illicitly, but again swiftly lost popularity due to a spate of fatal overdose cases.[16][17][18] A number of related compounds are known, and new derivatives have continued to appear on the designer drug market.[19][20][21][22][23][24]
List of substituted aminorex derivatives
| Structure | Common name | Chemical name | CAS number |
|---|---|---|---|
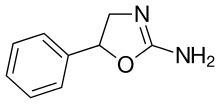 |
Aminorex | 5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 2207-50-3 |
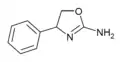 |
Rexamino | 4-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 52883-35-9 |
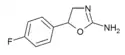 |
4'-Fluoroaminorex (4'-FAR) | 5-(4-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | 2967-77-3 |
| Clominorex | 5-(4-chlorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | 3876-10-6 | |
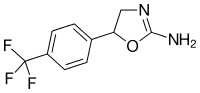 |
Fluminorex | 5-[4-(trifluoromethyl)phenyl]-4,5-dihydro-1,3-oxazol-2-amine | 720-76-3 |
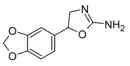 |
Methylenedioxyaminorex | 5-(3,4-methylenedioxyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 3865-98-3 |
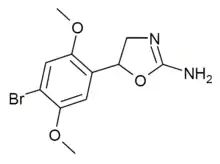 |
2C-B-aminorex (2C-B-AR) | 5-(2,5-dimethoxy-4-bromophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |
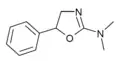 |
N,N-Dimethylaminorex (N,N-DMAR) | N,N-dimethyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 32968-41-5 |
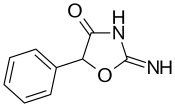 |
Pemoline | 2-amino-5-phenyl-1,3-oxazol-4(5H)-one | 2152-34-3 |
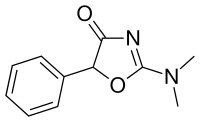 |
Thozalinone | 2-(dimethylamino)-5-phenyl-1,3-oxazol-4(5H)-one | 655-05-0 |
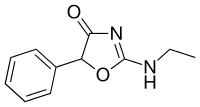 |
Fenozolone | 2-ethylamino-5-phenyl-1,3-oxazol-4-one | 15302-16-6 |
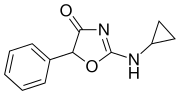 |
Cyclazodone | 2-(cyclopropylamino)-5-phenyl-1,3-oxazol-4-one | 14461-91-7 |
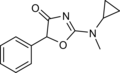 |
N-Methylcyclazodone | 2-(cyclopropyl(methyl)amino)-5-phenyl-1,3-oxazol-4-one | 14461-92-8 |
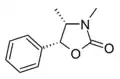 |
Ephedroxane [25] | (4S,5R)-3,4-dimethyl-5-phenyl-1,3-oxazolidin-2-one | 16251-46-0 |
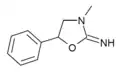 |
3-Methylaminorex | 3-methyl-5-phenyl-2-oxazolidinimine | 75343-73-6 |
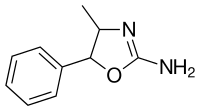 |
4-Methylaminorex (4-MAR) | 4-methyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 3568-94-3 |
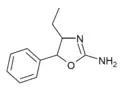 |
4-Ethylaminorex (4-EAR) | 4-ethyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 1364933-63-0 |
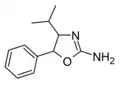 |
4-Isopropylaminorex | 4-isopropyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | |
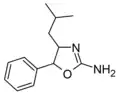 |
4-Isobutylaminorex | 4-(2-methylpropyl)-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | |
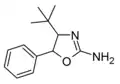 |
4-tert-butylaminorex | 4-(1,1-dimethylethyl)-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | |
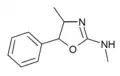 |
4,N-Dimethylaminorex (4,N-DMAR) | 4,5-dihydro-N,4-dimethyl-5-phenyl-2-oxazolamine | 2207-49-0 |
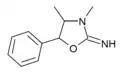 |
3,4-Dimethylaminorex (3,4-DMAR) | 3,4-dimethyl-5-phenyl-2-oxazolidinimine | 82485-31-2 |
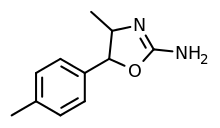 |
4,4'-Dimethylaminorex (4,4'-DMAR) | 4-methyl-5-(4-methylphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445569-01-6 |
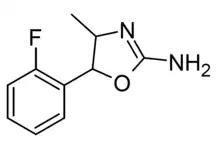 |
2'-Fluoro-4-methylaminorex (2F-MAR) | 4-methyl-5-(2-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |
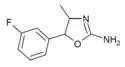 |
3'-Fluoro-4-methylaminorex (3F-MAR) | 4-methyl-5-(3-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |
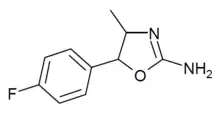 |
4'-Fluoro-4-methylaminorex (4F-MAR) | 4-methyl-5-(4-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1364933-64-1 |
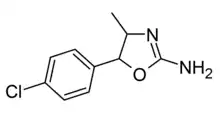 |
4'-Chloro-4-methylaminorex (4C-MAR) | 4-methyl-5-(4-chlorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |
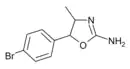 |
4'-Bromo-4-methylaminorex (4B-MAR) | 4-methyl-5-(4-bromophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |
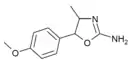 |
4'-Methoxy-4-methylaminorex (4'-MeO-4-MAR) | 4-methyl-5-(4-methoxyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445570-65-9 |
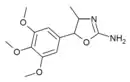 |
3',4',5'-Trimethoxy-4-methylaminorex (TM-4-MAR) | 4-methyl-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445571-92-5 |
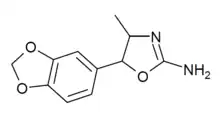 |
3',4'-Methylenedioxy-4-methylaminorex (MDMAR) | 4-methyl-5-(3,4-methylenedioyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445573-16-9 |
See also
References
- ↑ Meschino JA, Poos GI. 2-amino-5,6-dihydro-4H-1,3-oxazines and a process for their preparation. US Patent 3115494, 1961
- ↑ Poos GI. 2-amino-5-aryloxazoline products. US Patent 3161650, 1962
- ↑ Poos GI, Carson JR, Rosenau JD, Roszkowski AP, Kelley NM, Mcgowin J (May 1963). "2-Amino-5-aryl-2-oxazolines. Potent New Anorectic Agents". Journal of Medicinal Chemistry. 6 (3): 266–272. doi:10.1021/jm00339a011. PMID 14185981.
- ↑ Gurtner HP (1985). "Aminorex and pulmonary hypertension. A review". Cor et Vasa. 27 (2–3): 160–171. PMID 3928246.
- ↑ Davis FT, Brewster ME (March 1988). "A fatality involving U4Euh, a cyclic derivative of phenylpropanolamine". Journal of Forensic Sciences. 33 (2): 549–553. doi:10.1520/JFS11971J. PMID 3373171.
- ↑ Bunker CF, Johnson M, Gibb JW, Bush LG, Hanson GR (May 1990). "Neurochemical effects of an acute treatment with 4-methylaminorex: a new stimulant of abuse". European Journal of Pharmacology. 180 (1): 103–111. doi:10.1016/0014-2999(90)90597-y. PMID 1973111.
- ↑ Gaine SP, Rubin LJ, Kmetzo JJ, Palevsky HI, Traill TA (November 2000). "Recreational use of aminorex and pulmonary hypertension". Chest. 118 (5): 1496–1497. doi:10.1378/chest.118.5.1496. PMID 11083709.
- ↑ Meririnne E, Kajos M, Kankaanpää A, Koistinen M, Kiianmaa K, Seppälä T (August 2005). "Rewarding properties of the stereoisomers of 4-methylaminorex: involvement of the dopamine system". Pharmacology, Biochemistry, and Behavior. 81 (4): 715–724. doi:10.1016/j.pbb.2005.04.020. PMID 15982727. S2CID 21142560.
- ↑ Schmidt L, Scheffler H. Central nervous system stimulant. US Patent 2892753, 1957
- ↑ "Hardy RA, Howell CF, Quinones NQ. Method of producing central nervous system stimulation and anorexia. US Patent 3313688, 1964". Archived from the original on 2021-05-31. Retrieved 2019-06-14.
- ↑ "Guidicelli DP, Najer H. 5-phenyl-2-cyclopropylamino-4-oxazolinone, and process for making the same. US Patent 3609159, 1967". Archived from the original on 2021-05-31. Retrieved 2019-06-14.
- ↑ Marotta PJ, Roberts EA (May 1998). "Pemoline hepatotoxicity in children". The Journal of Pediatrics. 132 (5): 894–897. doi:10.1016/s0022-3476(98)70329-4. PMID 9602211.
- ↑ Safer DJ, Zito JM, Gardner JE (June 2001). "Pemoline hepatotoxicity and postmarketing surveillance". Journal of the American Academy of Child and Adolescent Psychiatry. 40 (6): 622–629. doi:10.1097/00004583-200106000-00006. PMID 11392339.
- ↑ Etwel FA, Rieder MJ, Bend JR, Koren G (2008). "A surveillance method for the early identification of idiosyncratic adverse drug reactions". Drug Safety. 31 (2): 169–180. doi:10.2165/00002018-200831020-00006. PMID 18217792. S2CID 19964105.
- ↑ Shader RI (April 2017). "Risk Evaluation and Mitigation Strategies (REMS), Pemoline, and What Is a Signal?". Clinical Therapeutics. 39 (4): 665–669. doi:10.1016/j.clinthera.2017.03.008. PMID 28366595.
- ↑ Brandt SD, Baumann MH, Partilla JS, Kavanagh PV, Power JD, Talbot B, et al. (2014). "Characterization of a novel and potentially lethal designer drug (±)-cis-para-methyl-4-methylaminorex (4,4'-DMAR, or 'Serotoni')". Drug Testing and Analysis. 6 (7–8): 684–695. doi:10.1002/dta.1668. PMC 4128571. PMID 24841869.
- ↑ Coppola M, Mondola R (July 2015). "4,4'-DMAR: chemistry, pharmacology and toxicology of a new synthetic stimulant of abuse". Basic & Clinical Pharmacology & Toxicology. 117 (1): 26–30. doi:10.1111/bcpt.12399. PMID 25819702.
- ↑ Maier J, Mayer FP, Luethi D, Holy M, Jäntsch K, Reither H, et al. (August 2018). "The psychostimulant (±)-cis-4,4'-dimethylaminorex (4,4'-DMAR) interacts with human plasmalemmal and vesicular monoamine transporters". Neuropharmacology. 138: 282–291. doi:10.1016/j.neuropharm.2018.06.018. PMID 29908239. S2CID 49274224.
- ↑ Russell BR, Beresford RA, Schmierer DM, McNaughton N, Clark CR (1995). "Stimulus properties of some analogues of 4-methylaminorex". Pharmacology, Biochemistry, and Behavior. 51 (2–3): 375–378. doi:10.1016/0091-3057(94)00407-a. PMID 7667356. S2CID 28367828.
- ↑ Zheng Y, Russell B, Schmierer D, Laverty R (January 1997). "The effects of aminorex and related compounds on brain monoamines and metabolites in CBA mice". The Journal of Pharmacy and Pharmacology. 49 (1): 89–96. doi:10.1111/j.2042-7158.1997.tb06758.x. PMID 9120777. S2CID 20224300.
- ↑ McLaughlin G, Morris N, Kavanagh PV, Power JD, Twamley B, O'Brien J, et al. (July 2015). "Synthesis, characterization, and monoamine transporter activity of the new psychoactive substance 3',4'-methylenedioxy-4-methylaminorex (MDMAR)". Drug Testing and Analysis. 7 (7): 555–564. doi:10.1002/dta.1732. PMC 5331736. PMID 25331619.
- ↑ Maier J, Mayer FP, Brandt SD, Sitte HH (October 2018). "DARK Classics in Chemical Neuroscience: Aminorex Analogues". ACS Chemical Neuroscience. 9 (10): 2484–2502. doi:10.1021/acschemneuro.8b00415. PMC 6287711. PMID 30269490.
- ↑ Fabregat-Safont D, Carbón X, Ventura M, Fornís I, Hernández F, Ibáñez M (June 2019). "Characterization of a recently detected halogenated aminorex derivative: para-fluoro-4-methylaminorex (4'F-4-MAR)". Scientific Reports. 9 (1): 8314. Bibcode:2019NatSR...9.8314F. doi:10.1038/s41598-019-44830-y. PMC 6549166. PMID 31165778.
- ↑ Rickli A, Kolaczynska K, Hoener MC, Liechti ME (May 2019). "Pharmacological characterization of the aminorex analogs 4-MAR, 4,4'-DMAR, and 3,4-DMAR". Neurotoxicology. 72: 95–100. doi:10.1016/j.neuro.2019.02.011. PMID 30776375. S2CID 73474963.
- ↑ Hikino H, Ogata K, Kasahara Y, Konno C (May 1985). "Pharmacology of ephedroxanes". Journal of Ethnopharmacology. 13 (2): 175–191. doi:10.1016/0378-8741(85)90005-4. PMID 4021515.