 | |
| Names | |
|---|---|
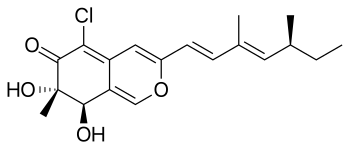
| Preferred IUPAC name
(7S,8R)-5-Chloro-3-[(1E,3E,5S)-3,5-dimethylhepta-1,3-dien-1-yl]-7,8-dihydroxy-7-methyl-7,8-dihydro-6H-2-benzopyran-6-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C19H23ClO4 | |
| Molar mass | 350.84 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Luteusin A is an azaphilone monoamine oxidase inhibitor produced by Talaromyces luteus.[1]
References
- ↑ Yoshida, E; Fujimoto, H; Yamazaki, M (1996). "Isolation of three new azaphilones, luteusins C, D, and E, from an ascomycete, talaromyces luteus". Chemical & Pharmaceutical Bulletin. 44 (2): 284–7. doi:10.1248/cpb.44.284. PMID 8998836.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.