This page provides supplementary chemical data on lycopene.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
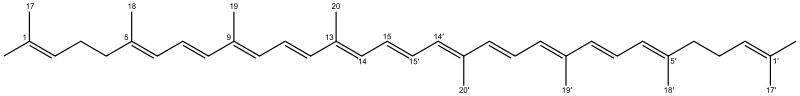
All-trans-lycopene with canonical numbering:
| Structure and properties | |
|---|---|
| Index of refraction, nD | ? |
| Dielectric constant, εr | ? ε0 at ? °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
Spectral data
To date, no X-ray crystal structure of lycopene has been reported.
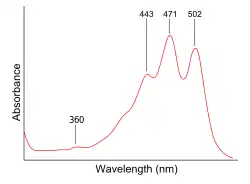
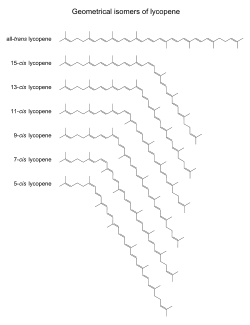
UV spectra of lycopene in hexane. A typical carotenoid, lycopene displays three absorbance maxima. A peak at 360 nm would indicate the presence of certain cis-isomers
| UV-Vis | |
|---|---|
| λmax | 443, 471, 502 nm in hexane |
| Extinction coefficient, ε | 1.72 × 105 L•mol−1•cm−1(at 502 nm)[3] |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
This box:
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References
- ↑ Chasse, Gregory A.; Mak, Melody L.; Deretey, Eugen; Farkas, Imre; Torday, Ladislaus L.; Papp, Julius G.; Sarma, Dittakavi S.R; Agarwal, Anita; Chakravarthi, Sujatha; Agarwal, Sanjiv; Rao, A.Venket (2001). "An ab initio computational study on selected lycopene isomers". Journal of Molecular Structure: Theochem. 571 (1–3): 27–37. doi:10.1016/S0166-1280(01)00424-9.
- ↑ Chasse, Gregory A.; Chasse, Kenneth P.; Kucsman, Arpad; Torday, Ladislaus L.; Papp, Julius G. (2001). "Conformational potential energy surfaces of a Lycopene model". Journal of Molecular Structure: Theochem. 571 (1–3): 7–26. doi:10.1016/S0166-1280(01)00413-4.
- ↑ For hexane:CH2Cl2 98:2 v/v
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.