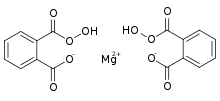
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Magnesium bis(2-carbonoperoxoylbenzoate) | |
| Other names
H48; MMPP | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.071.808 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C16H10MgO10 | |
| Molar mass | 386.551 g·mol−1 |
| Hazards | |
| Flash point | 173.4 °C (344.1 °F; 446.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Magnesium monoperoxyphthalate (MMPP) is a water-soluble peroxy acid used as an oxidant in organic synthesis. Its main areas of use are the conversion of ketones to esters (Baeyer-Villiger oxidation), epoxidation of alkenes (Prilezhaev reaction), oxidation of sulfides to sulfoxides and sulfones, oxidation of amines to produce amine oxides, and in the oxidative cleavage of hydrazones.[1]
Due to its insolubility in non-polar solvents MMPP has seen less use than the more widely used meta-chloroperoxybenzoic acid (mCPBA). Although work up procedures are more simply handled in polar solvents, usage of MMPP to oxidize nonpolar substrates in biphasic media combined with a phase transfer catalyst have been inefficient.[1] Despite this MMPP has certain advantages over mCPBA including a lower cost of production and increased stability.[1]
MMPP is also used as the active ingredient in certain surface disinfectants such as Dismozon Pur.[2] As a surface disinfectant MMPP exhibits a broad spectrum biocidal effect including inactivation of endospores.[3] Its wide surface compatibility enables its use on sensitive materials, such as plastic and rubber equipment used in hospitals. Additionally MMPP has been investigated as a potential antibacterial agent for mouthwashes and toothpaste.[4]
References
- 1 2 3 Carvalho, João F.S.; Silva, M. Manuel Cruz; Sá e Melo, M. Luisa (2009). "Highly efficient epoxidation of unsaturated steroids using magnesium bis(monoperoxyphthalate) hexahydrate". Tetrahedron. 65 (14): 2773–81. doi:10.1016/j.tet.2009.01.100.
- ↑ "Magnesium Monoperoxyphthalate". ndrugs. Retrieved 19 December 2013.
- ↑ Baldry, M.G.C. (1984). "The antimicrobial properties of magnesium monoperoxyphthalate hexahydrate". Journal of Applied Bacteriology. 57 (3): 499–503. doi:10.1111/j.1365-2672.1984.tb01416.x. PMID 6397460.
- ↑ Scully, Crispian; El-Kabir, Mohamed; Greenman, John; Porter, Stephen R.; Mutlu, Serdar; Barton, Ian; Adair, Richard (1999). "The effects of mouth rinses and dentifrice-containing magnesium monoperoxyphthalate (mmpp) on oral microflora, plaque reduction, and mucosa". Journal of Clinical Periodontology. 26 (4): 234–8. doi:10.1034/j.1600-051X.1999.260406.x. PMID 10223394.