 | |
| Names | |
|---|---|
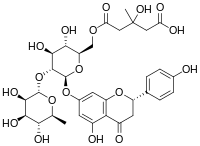
| IUPAC name
5-[[(2R,3S,4S,5R,6S)-3,4-Dihydroxy-6-[[(2S)-5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-2,3-dihydrochromen-7-yl]oxy]-5-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]methoxy]-3-hydroxy-3-methyl-5-oxopentanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C33H40O18 | |
| Molar mass | 724.665 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Melitidin is a flavanone glycoside. Melitidin was discovered in bergamot orange juice and exhibits statin-like properties in preclinical research.[1][2][3][4][5]
See also
References
- ↑ Di Donna, Leonardo; De Luca, Giuseppina; Mazzotti, Fabio; Napoli, Anna; Salerno, Raffaele; Taverna, Domenico; Sindona, Giovanni (2009). "Statin-like Principles of Bergamot Fruit: Isolation of 3-Hydroxymethylglutaryl Flavonoid Glycosides". Journal of Natural Products. 72 (7): 1352–1354. doi:10.1021/np900096w. PMID 19572741.
- ↑ Di Donna, Leonardo; Gallucci, Giselda; Malaj, Naim; Romano, Elvira; Tagarelli, Antonio; Sindona, Giovanni (2011). "Recycling of industrial essential oil waste: Brutieridin and Melitidin, two anticholesterolaemic active principles from bergamot albedo". Food Chemistry. 125 (2): 438–441. doi:10.1016/j.foodchem.2010.09.025. ISSN 0308-8146.
- ↑ Natalizia Miceli; M. R. Mondello; M. T. Monforte; V. Sdrafkakis; P. Dugo; M. L. Crupi; M. F. Taviano; R. De Pasquale; A. Trovato (2007). "Hypolipidemic Effects of Citrus bergamia Risso et Poiteau Juice in Rats Fed a Hypercholesterolemic Diet". J. Agric. Food Chem. 55 (27): 10671–10677. doi:10.1021/jf071772i. PMID 18038978.
- ↑ Monica Leopoldini; N. Malaj; M. Toscano; G. Sindona & N. Russo (2010). "On the Inhibitor Effects of Bergamot Juice Flavonoids Binding to the 3-Hydroxy-3-methylglutaryl-CoA Reductase (HMGR) Enzyme". J. Agric. Food Chem. 58 (19): 10768–10773. doi:10.1021/jf102576j. PMID 20843083.
- ↑ Vincenzo Mollace; I. Sacco; E. Janda; C. Malara; D. Ventrice; C. Colica; V. Visalli; S. Muscoli; S. Ragusa; C. Muscoli; D. Rotirotia; F. Romeo (2011). "Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies". Fitoterapia. 82 (3): 309–316. doi:10.1016/j.fitote.2010.10.014. PMID 21056640. PDF Archived 2012-04-02 at the Wayback Machine
Bibliography
- Zou, W; Wang Y; Liu H; Luo Y; Chen S; Su W (2013). "Melitidin: a flavanone glycoside from Citrus grandis 'Tomentosa'". Nat Prod Commun. 8 (4): 457–458. PMID 23738451.
- Mencherini, Teresa; Campone, Luca; Piccinelli, Anna Lisa; García Mesa, Milagros; Sánchez, Dulce María; Aquino, Rita Patrizia; Rastrelli, Luca (2013). "HPLC-PDA-MS and NMR Characterization of a Hydroalcoholic Extract of Citrus aurantium L. var.amara Peel with Antiedematogenic Activity". Journal of Agricultural and Food Chemistry. 61 (8): 1686–1693. doi:10.1021/jf302815t. ISSN 0021-8561. PMID 22957519.
- Barreca, Davide; Bellocco, Ersilia; Caristi, Corrado; Leuzzi, Ugo; Gattuso, Giuseppe (2011). "Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl)glycosyl flavanones and furocoumarins in Citrus aurantium L. juice". Food Chemistry. 124 (2): 576–582. doi:10.1016/j.foodchem.2010.06.076. ISSN 0308-8146.
- Di Donna, Leonardo; Taverna, Domenico; Mazzotti, Fabio; Benabdelkamel, Hicham; Attya, Mohamed; Napoli, Anna; Sindona, Giovanni (2013). "Comprehensive assay of flavanones in citrus juices and beverages by UHPLC–ESI-MS/MS and derivatization chemistry". Food Chemistry. 141 (3): 2328–2333. doi:10.1016/j.foodchem.2013.05.034. ISSN 0308-8146. PMID 23870965.
External links
 Media related to Melitidin at Wikimedia Commons
Media related to Melitidin at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.