 | |
| Names | |
|---|---|
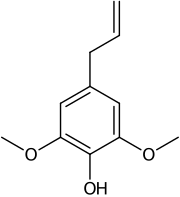
| IUPAC name
2,6-dimethoxy-4-prop-2-enylphenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.026.910 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H14O3 | |
| Molar mass | 194.230 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H317, H319, H335 | |
| P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Methoxyeugenol is a naturall occurring allylbenzene and eugenol derivative. It is found in toxic Japanese star anise pericarp[1] and leaves.[2] as well as in nutmeg crude extract but not in nutmeg essential oil.[3] It also activates PPAR-gamma in vivo.[4]
See also
References
- ↑ Howes MJ, Kite GC, Simmonds MS (July 2009). "Distinguishing chinese star anise from Japanese star anise using thermal desorption-gas chromatography-mass spectrometry". Journal of Agricultural and Food Chemistry. 57 (13): 5783–9. doi:10.1021/jf9009153. PMID 19507874.
- ↑ Koeduka T, Sugimoto K, Watanabe B, Someya N, Kawanishi D, Gotoh T, Ozawa R, Takabayashi J, Matsui K, Hiratake J (March 2014). "Bioactivity of natural O-prenylated phenylpropenes from Illicium anisatum leaves and their derivatives against spider mites and fungal pathogens". Plant Biology. 16 (2): 451–6. doi:10.1111/plb.12054. PMID 23889818.
- ↑ Oo T, Saiboonjan B, Srijampa S, Srisrattakarn A, Sutthanut K, Tavichakorntrakool R, Chanawong A, Lulitanond A, Tippayawat P (July 2021). "Inhibition of Bacterial Efflux Pumps by Crude Extracts and Essential Oil from Myristica fragrans Houtt. (Nutmeg) Seeds against Methicillin-Resistant Staphylococcus aureus". Molecules (Basel, Switzerland). 26 (15): 4662. doi:10.3390/molecules26154662. PMC 8348620. PMID 34361815.
- ↑ de Souza Basso B, Haute GV, OrtegaRibera M, Luft C, Antunes GL, Bastos MS, Carlessi LP, Levorse VG, Cassel E, Donadio MV, Santarém ER, GraciaSancho J, Rodrigues de Oliveira J (July 2021). "Methoxyeugenol deactivates hepatic stellate cells and attenuates liver fibrosis and inflammation through a PPAR-ɣ and NF-kB mechanism". Journal of Ethnopharmacology. 280: 114433. doi:10.1016/j.jep.2021.114433. hdl:10923/18540. PMID 34280502.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.