 | |
| Names | |
|---|---|
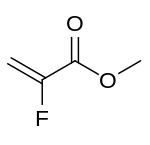
| Preferred IUPAC name
Methyl 2-fluoroprop-2-enoate | |
| Other names
Methyl 2-fluoroacrylate 2-Fluoro-2-propenoic acid methyl ester 2-Fluoroacrylic acid methyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.133.340 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H5FO2 | |
| Molar mass | 104.080 g·mol−1 |
| Appearance | transparent liquid |
| Density | 1.114 g/mL @ 20 °C |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 91 °C @ 750 mmHg |
| slightly soluble | |
Refractive index (nD) |
1.39 @ 20 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Methyl 2-fluoroacrylate (MFA) is a chemical compound classified as an acrylate ester. The molecular formula is C4H5FO2 and the molecular weight is 104.08. The systematic name of this chemical is methyl 2-fluoroprop-2-enoate. It is used in industrial chemistry to produce acrylate polymers with mechanical and optical properties,[1] and insect antifeedant 2-azabicyclo[2.1.1]hexane.[2]
Hazards
MFA is highly flammable and can be harmful if inhaled, in contact with skin, or if swallowed. It is irritating to eyes, respiratory system, and skin.[3]
References
- ↑ Fu, Boqiao; Cao, Zhen; Wu, Boying; Mao, Chongyang; Qin, Caiqin; Chen, Shigui (2022-08-12). "Novel facile method for the synthesis of methyl 2-fluoro-3-hydroxypropanoate from Claisen salts and formaldehyde in water". Phosphorus, Sulfur, and Silicon and the Related Elements. 197 (12): 1277–1283. doi:10.1080/10426507.2022.2097232. ISSN 1042-6507.
- ↑ Wiesmann, U. N.; DiDonato, S.; Herschkowitz, N. N. (1975-10-27). "Effect of chloroquine on cultured fibroblasts: release of lysosomal hydrolases and inhibition of their uptake". Biochemical and Biophysical Research Communications. 66 (4): 1338–1343. doi:10.1016/0006-291x(75)90506-9. ISSN 1090-2104. PMID 4.
- ↑ Material Safety Data Sheet. SynQuest Laboratories, Inc. Alachua, FL. Revised February, 2011
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.