 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl 2-oxopropanoate | |
| Identifiers | |
3D model (JSmol) |
|
| 1361953 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.081 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6O3 | |
| Molar mass | 102.089 g·mol−1 |
| Appearance | Colorless liquid |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 135 °C (275 °F; 408 K) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H226, H317, H318, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P271, P272, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P333+P313, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
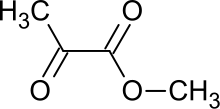
Methyl pyruvate is the organic compound with the formula CH3C(O)CO2CH3. This colorless liquid is the methyl ester of pyruvic acid. It has attracted interest as a prochiral precursor to alanine and lactic acid.[1] It is prepared by esterification of pyruvic acid.[2]
References
- ↑ Abdel-Magid, Ahmed F.; Carson, Kenneth G.; Harris, Bruce D.; Maryanoff, Cynthia A.; Shah, Rekha D. (1996). "Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures". Journal of Organic Chemistry. 61 (11): 3849–3862. doi:10.1021/JO960057X. PMID 11667239.
- ↑ A. Weissberger; C. J. Kibler (1944). "Methyl Pyruvate". Org. Synth. 24: 72. doi:10.15227/orgsyn.024.0072.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.