
The methylcitrate cycle, or the MCC, is the mechanism by which propionyl-CoA is formed, generated by β-oxidation of odd-chain fatty acids, and broken down to its final products, succinate and pyruvate.[1] The methylcitrate cycle is closely related to both the citric acid cycle and the glyoxylate cycle, in that they share substrates, enzymes and products.[2] The methylcitrate cycle functions overall to detoxify bacteria of toxic propionyl-CoA, and plays an essential role in propionate metabolism in bacteria. Incomplete propionyl-CoA metabolism may lead to the buildup of toxic metabolites in bacteria, and thus the function of the methylcitrate cycle is an important biological process.[3]
History
2-methylisocitric acid, an intermediate of the methylcitrate cycle, was first synthesized in 1886 as a mixture of four isomers. The pathway of the methylcitrate cycle was not discovered until 1973 in fungi, though it was not yet fully understood.[4] Originally, the methylcitrate cycle was thought to be present only in fungal species, such as Candida lipolytica and Aspergillus nidulans. In 1999, it was discovered that the methylcitrate cycle was also present in bacteria Salmonella enterica and Escherichia coli.[5] Much research has been done on the methylcitrate cycle's role in the development and function of various fungi and strains of bacteria, as well as its virulent properties in conjunction with the glyoxylate cycle.[6]
Steps
There are three basic steps in the methylcitrate cycle, as outlined below. Additionally, the mechanism is shown with its reactants, products, intermediates, and enzymes.[7]
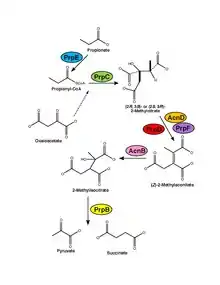
| Step | Substrates | Products | Enzymes | Reaction type | Comment |
|---|---|---|---|---|---|
| 1 | Propionate and oxaloacetate | 2-methylcitrate | Propionyl-CoA synthase and methylcitrate synthase | Aldol condensation | Propinate is first activated to propionyl-CoA by propionyl-CoA synthase, then propionyl-CoA is condensed with oxaloacetate by methylcitrate synthase to form the product, 2-methylcitrate. |
| 2 | 2-methylcitrate | 2-methylisocitrate | Methylcitrate dehydratase, methylaconitate cis-trans isomerase and aconitase | Isomerization | 2-methylcitrate is dehydrated to methylaconitate either by methylcitrate dehydratase, or by both methylcitrate dehydratase and methylaconitate cis-trans isomerase. It is then rehydrated by aconitase to form the product, 2-methylisocitrate. |
| 3 | 2-methylisocitrate | Succinate and pyruvate | methylisocitrate lyase | Cleavage | 2-methylisocitrate is cleaved by cycle-specific enzyme methylisocitrate lyase to form the final products, succinate and pyruvate. This cleavage completes the α-oxidation of the initial substrate, propionate, to its final product, pyruvate.[8] |
The major enzymes involved in this process are methylcitrate synthase (MCS) in step one, methylcitrate dehydratase (MCD) in step two, and 2-methylisocitrate lyase (MCL) in step three. The PrpC gene, which encodes for enzyme methylcitrate synthase in the first step of the methylcitrate cycle, is the gene responsible for propionate metabolism in the process. Without this gene, the methylcitrate cycle and ultimate metabolism would not occur, but rather catabolism.[9] The reaction of the methylcitrate cycle both overlaps and intertwines with the citric acid cycle and the glyoxylate cycle. Odd-chain fatty acids acetate and propionate are broken down by the β-oxidation cycle to form acetyl-CoA, which is further oxidized by the citric acid cycle, and propionyl-CoA, which is oxidized by the methylcitrate cycle. The substrate oxaloacetate is generated by the citric acid and glyoxylate cycles, and the product succinate is taken from the methylcitrate cycle to be used in the citric acid cycle.[10]

Products
One of the major products of the methylcitrate cycle is pyruvate. This pyruvate can be used by metabolic enzymes for energy and biomass formation. [11] The other major product, succinate, is used in the citric acid cycle and helps to carry the reaction forward and restarts the cycle. Succinate is used by the citric acid and glyoxylate cycles to generate oxaloacetate, one of the key substrates necessary to begin the methylcitrate cycle.
References
- ↑ Upton AM, McKinney JD (2007). Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology. 153 (12): 3973-3982. doi:10.1099/mic.0.2007/011726-0.
- ↑ Dolan SK, Wijaya A, Geddis SM, Spring DR, Silva-Rocha R, Welch M (2018). Loving the poison: the methylcitrate cycle and bacterial pathogenesis. Microbiology. 164 (3): 251-259. doi:10.1099/mic.0.000604.
- ↑ Upton AM, McKinney JD (2007). Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology. 153 (12): 3973-3982. doi:10.1099/mic.0.2007/011726-0.
- ↑ Tabuchi T, Hara S (1974). Production of 2-Methylisocitric Acid from n- Paraffins by Mutants of Candida lipolytica. Agricultural and Biological Chemistry. 38 (5): 1105-1106. doi:10.1080/00021369.1974.10861293.
- ↑ Dolan SK, Wijaya A, Geddis SM, Spring DR, Silva-Rocha R, Welch M (2018). Loving the poison: the methylcitrate cycle and bacterial pathogenesis. Microbiology. 164 (3): 251-259. doi:10.1099/mic.0.000604.
- ↑ Lee SH, Han YK, Yun SH, Lee YW (2009). Roles of the Glyoxylate and Methylcitrate Cycles in Sexual Development and Virulence in the Cereal Pathogen Gibberella zeae. Eukaryotic Cell. 8 (8): 1155-1164. doi: 10.1128/EC.00335-08.
- ↑ Dolan SK, Wijaya A, Geddis SM, Spring DR, Silva-Rocha R, Welch M (2018). Loving the poison: the methylcitrate cycle and bacterial pathogenesis. Microbiology. 164 (3): 251-259. doi:10.1099/mic.0.000604.
- ↑ Domin N, Wilson D, Brock M (2009). Methylcitrate cycle activation during adaptation of Fusarium solani and Fusarium vericillioides to propionyl-CoA-generating carbon sources. Microbiology. 155 (12): 3903-3912. doi: 10.1099/mic.0.031781-0.
- ↑ Luo H, Zhou D, Liu X, Nie Z, Quiroga-Sánchez DL, Chang Y (2016). Production of 3-Hydroxypropionic Acid via the Propionyl-CoA Pathway Using Recombinant Escherichia coli Strains. PLOS ONE. 11 (5): 1-13. doi:10.1371/journal.pone.0156286.
- ↑ Dolan SK, Wijaya A, Geddis SM, Spring DR, Silva-Rocha R, Welch M (2018). Loving the poison: the methylcitrate cycle and bacterial pathogenesis. Microbiology. 164 (3): 251-259. doi:10.1099/mic.0.000604.
- ↑ Domin N, Wilson D, Brock M (2009). Methylcitrate cycle activation during adaptation of Fusarium solani and Fusarium vericillioides to propionyl-CoA-generating carbon sources. Microbiology. 155 (12): 3903-3912. doi: 10.1099/mic.0.031781-0.
- ↑ Upton, AM; McKinney, JD (2007). "Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis". Microbiology. 153 (12): 3973–3982. doi:10.1099/mic.0.2007/011726-0. PMID 18048912.
- ↑ Dolan, SK; Wijaya, A; Geddis, SM; Spring, DR; Silva-Rocha, R; Welch, M (2018). "Loving the poison: the methylcitrate cycle and bacterial pathogenesis". Microbiology. 164 (3): 251–259. doi:10.1099/mic.0.000604. PMID 29458664.
- ↑ Domin, N; Wilson, D; Brock, M (2009). "Methylcitrate cycle activation during adaptation of Fusarium solani and Fusarium vericillioides to propionyl-CoA-generating carbon sources". Microbiology. 155 (12): 3903–3912. doi:10.1099/mic.0.031781-0. PMID 19661181.
- ↑ Luo, H; Zhou, D; Liu, X; Nie, Z; Quiroga-Sánchez, DL; Chang, Y (2016). "Production of 3-Hydroxypropionic Acid via the Propionyl-CoA Pathway Using Recombinant Escherichia coli Strains". PLOS ONE. 11 (5): 1–13. Bibcode:2016PLoSO..1156286L. doi:10.1371/journal.pone.0156286. PMC 4882031. PMID 27227837.
- ↑ Tabuchi, T; Hara, S (1974). "Production of 2-Methylisocitric Acid from n- Paraffins by Mutants of Candida lipolytica". Agricultural and Biological Chemistry. 38 (5): 1105–1106. doi:10.1080/00021369.1974.10861293. Retrieved 4 December 2020.
- ↑ Lee, SH; Han, YK; Yun, SH; Lee, YW (2009). "Roles of the Glyoxylate and Methylcitrate Cycles in Sexual Development and Virulence in the Cereal Pathogen Gibberella zeae". Eukaryotic Cell. American Society for Microbiology. 8 (8): 1155–1164. doi:10.1128/EC.00335-08. PMC 2725564. PMID 19525419. S2CID 2150671.