 | |
| Names | |
|---|---|
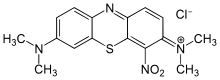
| IUPAC name
[7-(dimethylamino)-4-nitrophenothiazin-3-ylidene]-dimethylazanium
chloride | |
| Other names
Basic Green 5; CI 52020 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.018.392 |
| MeSH | C028673 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H17ClN4O2S | |
| Molar mass | 364.85 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Methylene green is a heterocyclic aromatic chemical compound similar to methylene blue. It is used as a dye.[1] It functions as a visible light-activated photocatalyst in organic synthesis.[2]
References
- ↑ Senior W (November 1969). "Staining of animal tissues with the dye base of methylene green in benzene to facilitate identification and selection of material". Stain Technol. 44 (6): 269–71. doi:10.3109/10520296909063364. PMID 4187577.
- ↑ Rogers, David A.; Gallegos, Jillian M.; Hopkins, Megan D.; Lignieres, Austin A.; Pitzel, Amy K.; Lamar, Angus A. (September 2019). "Visible-light photocatalytic activation of N-chlorosuccinimide by organic dyes for the chlorination of arenes and heteroarenes". Tetrahedron. 75 (36): 130498. doi:10.1016/j.tet.2019.130498.
External links
- methylene green (at stainsfile)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.