 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methylene bis(dibutylcarbamodithioate) | |
| Other names
Methylene Dibutyldithiocarbamate | |
| Identifiers | |
PubChem CID |
|
| UNII | |
| Properties | |
| C19H38N2S4 | |
| Molar mass | 422.77 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
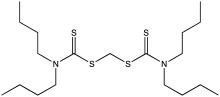
Methylenebis(dibutyldithiocarbamate) is the organosulfur compound with the formula CH2(SC(S)NBu2)2 (Bu = C4H9). It is a derivative of dibutyldithiocarbamate that is used as an additive to various lubricants, both as an antioxidant and to prevent metal surfaces.[1] It is prepared by alkylation of the dithiocarbamate with dichloromethane.[2] Although it is described as colored, simple esters of dithiocarbamate are typically colorless.[3]
References
- ↑ Theo Mang; Jürgen Braun; Wilfried Dresel; Jürgen Omeis (2011). "Lubricants, 2. Components". Ullmanns Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.o15_o04. ISBN 978-3-527-30673-2.
- ↑ Cespedes, Carlos; Vega, Juan C. (1994). "Reactions of Dichloromethane with Thioanions. 1. Preparation of Bis(N,N-dialkylthiocarbamoylthio)methanes". Phosphorus, Sulfur and Silicon and the Related Elements. 90 (1–4): 155–8. doi:10.1080/10426509408016397.
- ↑ John R. Grunwell (1970). "Reaction of Grignard reagents with tetramethylthiuram disulfide [yielding dithiocarbamates]". J. Org. Chem. 35 (5): 1500–1501. doi:10.1021/jo00830a052.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.