Michael E. Jung | |
|---|---|
Jung in 2016 | |
| Born | May 14, 1947 New Orleans |
| Occupation | Professor |
Michael E. Jung is a Professor of Chemistry in the Department of Chemistry and Biochemistry at the University of California at Los Angeles.
Michael Jung was born May 14, 1947, in New Orleans, Louisiana.[1]
Early life and education
Jung received a B.A. from Rice University in Houston, Texas, in 1969 and a Ph.D. from Columbia University in New York City in 1973 where he did research with Gilbert Stork.
Career
Jung then obtained a NATO Postdoctoral Fellowship to work with Albert Eschenmoser at the Eidgenössische Technische Hochschule in Zürich, Switzerland. In 1974, he joined the faculty at UCLA, where he has spent his career. In 1979 Jung was awarded a Sloan research fellowship.[2]
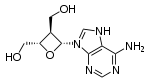
Jung's research is focused on the development of new reactions for organic synthesis, including the Jung "non-aldol aldol" protocol, an alternate method for obtaining aldol products without using the classical aldol reaction.[3] He has also developed chemical syntheses for a variety of natural products with antitumor and antiviral properties including tedanolides,[4][5][6] oxetanocin,[7] halomons,[8] and xestobergsterol.[9] Other research interests include the bridged Robinson annulation and the mixed Lewis acid Diels-Alder process.[10]

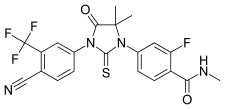
Jung's research group developed an antagonist of the androgen receptor enzalutamide, which is a pharmaceutical drug used for the treatment of hormone refractory prostate cancer.[11][12] An analog of enzalutamide, apalutamide, was also FDA approved.
Awards
- 2022 IUPAC-Richter Prize
- 2016 Glenn T. Seaborg Medal, UCLA[13]
- 2016 Richard C. Tolman Award, Southern California Section of the American Chemical Society[14]
- 2015 American Association for Cancer Research AACR Awards Team Science Award[15]
- 1995 American Chemical Society Arthur C. Cope Scholar Award[16]
References
- ↑ UCLA, Department of Chemistry and Biochemistry
- ↑ "Past Fellows". sloan.org.
- ↑ Research Interests, Organic Chemistry Faculty, UCLA
- ↑ Jung, Michael E.; Yoo, Dongwon (January 2008). "Synthesis of the C1–C12 fragment of the tedanolides. Selective hydroboration–protonation of allylic alcohol approach". Tetrahedron Letters. 49 (5): 816–819. doi:10.1016/j.tetlet.2007.11.181.
- ↑ Jung, Michael E.; Lee, Christopher P. (February 2001). "Synthesis of a Fully Functionalized Protected C1−C11 Fragment for the Synthesis of the Tedanolides". Organic Letters. 3 (3): 333–336. doi:10.1021/ol000329p. PMID 11428007.
- ↑ Jung, Michael E; Lee, Christopher P (December 2000). "Use of the non-aldol aldol process in the synthesis of the C1–C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans". Tetrahedron Letters. 41 (50): 9719–9723. doi:10.1016/s0040-4039(00)01726-3.
- ↑ Jung, Michael E.; Nichols, Christopher J. (October 1996). "Highly stereoselective synthesis of trans,trans-4-aryl-2,3-oxetanedimethanols: Preparation of oxetanocin a analogues". Tetrahedron Letters. 37 (43): 7667–7670. doi:10.1016/0040-4039(96)01720-0.
- ↑ Jung, Michael E.; Parker, Michael H. (1 October 1997). "Synthesis of Several Naturally Occurring Polyhalogenated Monoterpenes of the Halomon Class1". The Journal of Organic Chemistry. 62 (21): 7094–7095. doi:10.1021/jo971371+. ISSN 0022-3263. PMID 11671809.
- ↑ Jung, Michael E; Johnson, Ted W (February 2001). "First total synthesis of xestobergsterol A and active structural analogues of the xestobergsterols". Tetrahedron. 57 (8): 1449–1481. doi:10.1016/s0040-4020(00)01086-3.
- ↑ Current Research Interest, UCLA
- ↑ "New Prostate Cancer Agent Class: Rational design leads to novel drug candidate, now in clinical trials", Chemical and Engineering News, September 22, 2008, Volume 86, Number 38 pp. 84-87
- ↑ UCLA Chemistry and Biochemistry
- ↑ "UCLA Glenn T. Seaborg Symposium - Previous Recipients". www.seaborg.ucla.edu.
- ↑ "2015 Michael E. Jung, UCLA". SCALACS. 24 March 2016.
- ↑ "AACR Team Science Award". www.aacr.org.
- ↑ "Arthur C. Cope Scholar Awards". American Chemical Society.