 | |
| Clinical data | |
|---|---|
| Pronunciation | /moʊˈlɪndoʊn/ moh-LIN-dohn |
| Trade names | Moban |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a682238 |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| Drug class | Typical antipsychotic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 1.5 hours |
| Excretion | Minor, renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.254.109 |
| Chemical and physical data | |
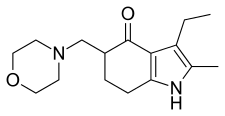
| Formula | C16H24N2O2 |
| Molar mass | 276.380 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Molindone, sold under the brand name Moban, is an antipsychotic which is used in the United States in the treatment of schizophrenia.[1][2] It works by blocking the effects of dopamine in the brain, leading to diminished symptoms of psychosis. It is rapidly absorbed when taken orally.
It is sometimes described as a typical antipsychotic,[3] and sometimes described as an atypical antipsychotic.[4]
Molindone was discontinued by its original supplier, Endo Pharmaceuticals, on January 13, 2010.[5]
Availability and marketing in the USA
After having been produced and subsequently discontinued by Core Pharma in 2015–2017, molindone is available again from Epic Pharma effective December, 2018.[6]
Adverse effects
The side effect profile of molindone is similar to that of other typical antipsychotics. Unlike most antipsychotics, however, molindone use is associated with weight loss.[4][7]
Chemistry
Synthesis
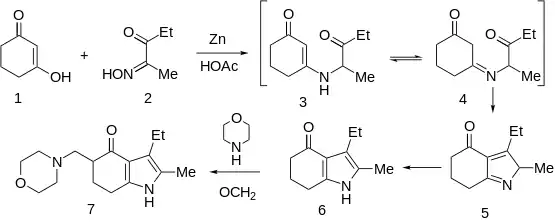
Condensation of oximinoketone 2 (from nitrosation of 3-pentanone), with cyclohexane-1,3-dione (1) in the presence of zinc and acetic acid leads directly to the partly reduced indole derivative 6. The transformation may be rationalized by assuming as the first step, reduction of 2 to the corresponding α-aminoketone. Conjugate addition of the amine to 1 followed by elimination of hydroxide (as water) would give ene-aminoketone 3. This enamine may be assumed to be in tautomeric equilibrium with imine 4. Aldol condensation of the side chain carbonyl group with the doubly activated ring methylene group would then result in cyclization to pyrrole 5; simple tautomeric transformation would then give the observed product. Mannich reaction of 6 with formaldehyde and morpholine gives the tranquilizer molindone (7).
See also
References
- ↑ "molindone". F.A. Davis Company.
- ↑ "Molindone".
- ↑ Aparasu RR, Jano E, Johnson ML, Chen H (October 2008). "Hospitalization risk associated with typical and atypical antipsychotic use in community-dwelling elderly patients". Am J Geriatr Pharmacother. 6 (4): 198–204. doi:10.1016/j.amjopharm.2008.10.003. PMID 19028375.
- 1 2 Bagnall A, Fenton M, Kleijnen J, Lewis R (2007). Bagnall AM (ed.). "Molindone for schizophrenia and severe mental illness". Cochrane Database Syst Rev (1): CD002083. doi:10.1002/14651858.CD002083.pub2. PMID 17253473.
- ↑ "Drugs to be Discontinued". www.fda.gov. Archived from the original on 2009-06-03.
- ↑ "NEWS". www.epic-pharma.com. Retrieved 2018-12-12.
- ↑ Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ (November 1999). "Antipsychotic-induced weight gain: a comprehensive research synthesis". The American Journal of Psychiatry. 156 (11): 1686–1696. doi:10.1176/ajp.156.11.1686. PMID 10553730. S2CID 38635470.
- ↑ SCHOEN KARL, J PACHTER IRWIN; BE 670798 (1965 to Endo Lab).
- ↑ Irwin J Pachter, Karl Schoen, U.S. Patent 3,491,093 (1970 to Endo Lab).
- ↑ Martin Hanbauer, et al. WO2014042688 (Supernus Pharmaceuticals Inc).