| |
 | |
| Clinical data | |
|---|---|
| Other names | EBS-101; PSYRX-101; SCH-39166 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H20ClNO |
| Molar mass | 313.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ecopipam (development codes SCH-39166, EBS-101, and PSYRX-101) is a dopamine antagonist which is under development for the treatment of Lesch-Nyhan syndrome, Tourette's syndrome, speech disorders, and restless legs syndrome.[1] It is taken by mouth.[2]
Ecopipam acts as a selective dopamine D1 and D5 receptor antagonist.[1] It is orally active, has an elimination half-life of 10 hours, crosses the blood–brain barrier, and substantially occupies brain dopamine receptors.[2][3] Side effects of ecopipam may include depression, anxiety, fatigue, sedation, somnolence, insomnia, headaches, muscle twitching, and suicidal ideation, among others.[4][5][2] It appears to lack the typical extrapyramidal effects like tardive dyskinesia that occur with D2 receptor antagonists.[2]
Ecopipam is an experimental drug and has not been approved for medical use.[1] As of April 2022, it is in phase 3 trials for Lesch-Nyhan syndrome, phase 2 trials for Tourette's syndrome and speech disorders, and phase 2/phase 1 trials for restless legs syndrome.[1] The drug was also under development for the treatment of cocaine-related disorders, obesity, and schizophrenia, but development for these indications was discontinued.[1]
Pharmacology
Pharmacodynamics
Ecopipam is a selective dopamine D1 and D5 receptor antagonist.[6] It shows little affinity for either dopamine D2-like or 5-HT2 receptors.[6]
Clinical trials
Based on its profile in animal models, ecopipam was first studied as a treatment for schizophrenia but showed no efficacy.[7][8] Side effects including sedation, restlessness, vomiting, and anxiety were generally rated mild. There were no reports of Parkinsonian-like extrapyramidal symptoms typically seen with D2 antagonists.
Human clinical studies also showed that ecopipam was an effective antagonist of the acute euphoric effects of cocaine.[9] However, the effect did not persist following repeated administration.[10]
Researchers have postulated that dopamine via D1 receptors in the mesolimbic system is involved with rewarded behaviors and pleasure.[11] One such behavior is eating, and ecopipam has been shown in a large clinical study to be an effective treatment for obesity.[12] However, reports of mild-to-moderate, reversible anxiety and depression made it unsuitable for commercialization as an anti-obesity drug, and its development was stopped for that indication.[13]
As of 2021, Emalex Biosciences is investigating its potential use for central nervous system disorders.[14] Open-label studies have found ecopipam to reduce gambling behaviors in subjects with pathological gambling[15] and to decrease the motor and vocal tics in adults with Tourette syndrome.[16] A subsequent double-blind placebo-controlled study in pediatric subjects confirmed ecopipam's ability to ameliorate the motor and vocal symptoms seen in patients with Tourette's syndrome.[17] Ecopipam is currently in a phase 2/3 clinical trial for the treatment of Tourette's syndrome in children ages 7 to 17.[18]
Ecopipam is additionally under development for the treatment of Lesch–Nyhan syndrome (phase 3) and restless legs syndrome (phase 1/2).[1]
Ecopipam is an investigational first-in-class drug being evaluated for the treatment of childhood-onset fluency disorder (stuttering) in adults. It is under development for the treatment of stuttering (phase 2).[19] There are currently no U.S. Food and Drug Administration approved medications for the treatment of stuttering.[19]
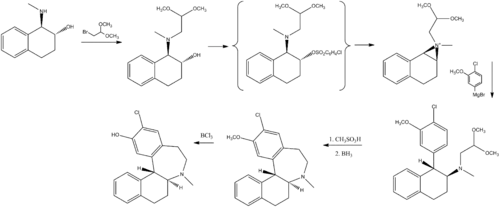
Chemistry
Chemically, ecopipam is a synthetic benzazepine derivative. It can be synthesized from a simple tetralin derivative:[20]
References
- 1 2 3 4 5 6 "Ecopipam - Emalex Biosciences". AdisInsight. Springer Nature Switzerland AG.
- 1 2 3 4 Khasnavis T, Torres RJ, Sommerfeld B, Puig JG, Chipkin R, Jinnah HA (July 2016). "A double-blind, placebo-controlled, crossover trial of the selective dopamine D1 receptor antagonist ecopipam in patients with Lesch-Nyhan disease". Molecular Genetics and Metabolism. 118 (3): 160–166. doi:10.1016/j.ymgme.2016.04.012. PMID 27179999.
- ↑ Karlsson P, Sedvall G, Halldin C, Swahn CG, Farde L (October 1995). "Evaluation of SCH 39166 as PET ligand for central D1 dopamine receptor binding and occupancy in man". Psychopharmacology. 121 (3): 300–308. doi:10.1007/BF02246067. PMID 8584610. S2CID 12659381.
- ↑ Nathan PJ, O'Neill BV, Napolitano A, Bullmore ET (October 2011). "Neuropsychiatric adverse effects of centrally acting antiobesity drugs". CNS Neuroscience & Therapeutics. 17 (5): 490–505. doi:10.1111/j.1755-5949.2010.00172.x. PMC 6493804. PMID 21951371.
Recently a study reported findings from human phase 2 and phase 3 clinical trials examining the potential of the D1/D5 receptor antagonist, ecopipam, to enhance and maintain weight loss in obese patients [61]. While these studies showed promising weight loss in both phase 2 and phase 3, there were unexpected treatment related neuropsychiatric adverse events (ecopipam 31% vs. placebo 15%) in the phase 3 clinical trials (that were not observed in the phase 2 studies) and as a consequence phase 3 studies were discontinued. The neuropsychiatric adverse events included depression (ecopipam 16% vs. placebo 6%), anxiety (ecopipam 15% vs. placebo 6%), suicidal ideation (ecopipam 2% vs. placebo 1%), insomnia (ecopipam 17% vs. placebo 7%), fatigue (ecopipam 15% vs. placebo 6%), and somnolence (ecopipam 15% vs. placebo 4%). Psychiatric adverse events also accounted for more than half of the discontinuations because of treatment related adverse effects in the ecopipam group.
- ↑ Gilbert DL, Budman CL, Singer HS, Kurlan R, Chipkin RE (2014). "A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome". Clinical Neuropharmacology. 37 (1): 26–30. doi:10.1097/WNF.0000000000000017. PMID 24434529. S2CID 24829565.
- 1 2 Chipkin RE, Iorio LC, Coffin VL, McQuade RD, Berger JG, Barnett A (December 1988). "Pharmacological profile of SCH39166: a dopamine D1 selective benzonaphthazepine with potential antipsychotic activity". The Journal of Pharmacology and Experimental Therapeutics. 247 (3): 1093–1102. PMID 2905002.
- ↑ Karlsson P, Smith L, Farde L, Härnryd C, Sedvall G, Wiesel FA (October 1995). "Lack of apparent antipsychotic effect of the D1-dopamine receptor antagonist SCH39166 in acutely ill schizophrenic patients". Psychopharmacology. 121 (3): 309–316. doi:10.1007/bf02246068. PMID 8584611. S2CID 23909094.
- ↑ Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, et al. (October 1995). "Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia". Psychopharmacology. 121 (3): 317–322. doi:10.1007/bf02246069. PMID 8584612. S2CID 21837432.
- ↑ Haney M, Ward AS, Foltin RW, Fischman MW (June 2001). "Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans". Psychopharmacology. 155 (4): 330–337. doi:10.1007/s002130100725. PMID 11441422. S2CID 973041.
- ↑ Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL (June 2001). "Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine". Psychopharmacology. 155 (4): 338–347. doi:10.1007/s002130100724. PMID 11441423. S2CID 854984.
- ↑ Baik JH (October 11, 2013). "Dopamine signaling in reward-related behaviors". Frontiers in Neural Circuits. 7: 152. doi:10.3389/fncir.2013.00152. PMC 3795306. PMID 24130517.
- ↑ Astrup A, Greenway FL, Ling W, Pedicone L, Lachowicz J, Strader CD, Kwan R (July 2007). "Randomized controlled trials of the D1/D5 antagonist ecopipam for weight loss in obese subjects". Obesity. 15 (7): 1717–1731. doi:10.1038/oby.2007.205. PMID 17636090. S2CID 11657547.
- ↑ Coulter AA, Rebello CJ, Greenway FL (July 2018). "Centrally Acting Agents for Obesity: Past, Present, and Future". Drugs. 78 (11): 1113–1132. doi:10.1007/s40265-018-0946-y. PMC 6095132. PMID 30014268.
- ↑ "Research & Development". Emalex Biosciences.
- ↑ Grant JE, Odlaug BL, Black DW, Fong T, Davtian M, Chipkin R, Kim SW (August 2014). "A single-blind study of 'as-needed' ecopipam for gambling disorder". Annals of Clinical Psychiatry. 26 (3): 179–186. PMID 25166480.
- ↑ Gilbert DL, Budman CL, Singer HS, Kurlan R, Chipkin RE (January–February 2014). "A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome". Clinical Neuropharmacology. 37 (1): 26–30. doi:10.1097/WNF.0000000000000017. PMID 24434529. S2CID 24829565.
- ↑ Gilbert DL, Murphy TK, Jankovic J, Budman CL, Black KJ, Kurlan RM, et al. (August 2018). "Ecopipam, a D1 receptor antagonist, for treatment of tourette syndrome in children: A randomized, placebo-controlled crossover study". Movement Disorders. 33 (8): 1272–1280. doi:10.1002/mds.27457. PMID 30192018. S2CID 52169188.
- ↑ Clinical trial number NCT04007991 for "Multicenter, Placebo-Controlled, Double-Blind, Randomized, Parallel-Group, Phase 2b Study to Evaluate the Efficacy and Safety of Ecopipam in Children and Adolescents With Tourette's Syndrome" at ClinicalTrials.gov
- 1 2 "First Patient Dosed in Emalex Biosciences Phase 2 Clinical Trial for Stuttering". Emalex Biosciences. 15 December 2020.
- ↑ Hou D, Schumacher D (November 2001). "The selection of a commercial route for the D1 antagonist Sch-39166". Current Opinion in Drug Discovery & Development. 4 (6): 792–799. PMID 11899619.