| Nerodia clarkii | |
|---|---|
 | |
| N. clarkii compressicauda, red color phase | |
 | |
| N. clarkii compressicauda, normal color phase | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Colubridae |
| Genus: | Nerodia |
| Species: | N. clarkii |
| Binomial name | |
| Nerodia clarkii | |
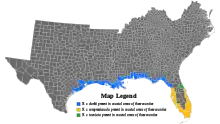 | |
| Nerodia clarkii resides in coastal areas in shaded counties.
Gulf salt marsh snake:
| |
| Synonyms[3] | |
| |
Nerodia clarkii, commonly known as the salt marsh snake, is a species of semi-aquatic, nonvenomous, colubrid snake found in the southeastern United States. Their range extends along the brackish salt marshes of the Gulf of Mexico and the Atlantic Coast from Texas to Florida, with an additional population in northern Cuba.[2] Different subspecies of this snake are primarily identified via color patterns on each snake's belly, or anterior.[4]
Etymology
The specific name, clarkii, is in honor of American surveyor and naturalist John Henry Clark (1830–1885).[5]
Description and subspecies
General description
Salt marsh snakes grow to a total length (including tail) of 15–30 inches (38–76 cm). They are also highly variable in both pattern and coloration. Found most commonly in salt marshes, this snake inhabits brackish and saltwater habitats; it is also found hiding in crab burrows.[6] Though salt marsh snakes are common throughout the territory they inhabit, they have a tendency to be wary and secretive, so they are rarely seen.[7] All members of this species reproduce via live birth, and all are semi-aquatic. Furthermore, all forms of this species may be distinguished as having either 21 or 23 rows of scales.[8]
The seawater they inhabit exerts a continual draw on their tissue's electrolyte balance, due to osmosis. Its scaly reptilian skin acts as a barrier against external dehydration, but, if ingested, seawater draws the less-salty fluid from blood and tissues into the stomach. N. clarkii is the only species to establish itself in this saline niche, drinking only rainwater when it is available, and at other times swallowing nothing but prey animals with the same diluted body fluids as their own.[8] All water species of snake (including N. clarkii) are typically considered to be non-venomous, though they do employ a complex series of enzymes in their saliva, resulting in some inflammation and edema to those who have been bitten.[7]
Subspecies
The salt marsh snake has three distinct subspecies, all of which were first discovered and classified in the mid to late 1800s. They are as follows:
Gulf salt marsh snake (N. c. clarkii)
This is perhaps the most prominent of the three races of N. clarkii, and is certainly the one with the furthest range. Populations of the Gulf salt marsh snake natively range from the vicinity of Corpus Christi, Texas, to the Gulf Hammock region of Florida. They are characterized by their prominent stripes; members of this race can be gray, tan or yellow, but all exhibit four brown to black longitudinal stripes which run from the back of their neck to their tail. The belly is reversed in color from the dorsum, and is reddish with a central light line of cream-colored oval blotches, often flanked by a row of pale spots. The scales are in either 21 or 23 rows, and the anal plate is divided.[8] There is little ontogenetic difference between juvenile to fully adult snakes.[7] Individuals subsist on a diet of primarily fish, and especially shallows-living species such as killifish and small mullet, as well as crayfish and shrimp.[8] Members of this race are primarily nocturnal during hot summer nights, but may be found basking and foraging during daylight hours in cool weather.[7] Sexual maturity is reached at three years.[9]
.jpg.webp)
Mangrove salt marsh snake (N. c. compressicauda)
The mangrove salt marsh snake's native range of populations is in Florida, from Tampa Bay south to Miami and northward along the Atlantic coast to the vicinity of Cape Canaveral. Within this range, N. c. compressicauda primarily inhabits inundated estuary forests of buttonwood and red mangroves, so as to minimize competition with Florida's numerous freshwater natricines. A study found sheepshead minnows to be the most common of the small estuarine fish preyed upon by this subspecies; it also described this subspecies as a very sedentary predator that remained motionless until ripples in the water close to their bodies triggered predatory behavior. The study also found a tendency toward minimal foraging.[8] This subspecies exhibits many colors and patterns and can be gray, green, or tan with darker banding or may even be solid reddish orange or straw yellow. It is also known to intergrade with all other types of salt marsh snake, complicating the identification process.[7] There are two primary forms: a gray-green color morph, and an orange variant. The gray-green variant has a dark crossbanded olive gray dorsum (some individuals that exhibit this color scheme have partial lateral striping on the forebody) with a clouded, gray-green venter. Adults of the orange phase, on the other hand, have an unmarked orangish back and sides; juveniles of this phase are orange with dark dorsal crossbands. All orange snakes in this subspecies exhibit a pale yellow venter, regardless of age.[8] This race is nocturnal.[10]
Atlantic salt marsh snake (N. c. taeniata)
A third subspecies, the Atlantic salt marsh snake (N. c. taeniata), in its pure form is restricted to a small stretch of coastline in Volusia and Indian River Counties, Florida. However, intergrades between it and the mangrove salt marsh snake extend a county or two southward of Volusia county.[7] Much of its habitat has been lost due to commercial development of the area's ocean coastline, such as excessive filling and development in salt marshes.[8][11] In fact, habitat loss is so severe for this subspecies that it has been listed as a Threatened Species by the US Fish and Wildlife Service,[12] having received this designation in 1977.[13] Members of this race have a tendency to be smaller than the other two races, with a record size of only 24 inches (61 cm).[11] Atlantic salt marsh snakes have a color pattern of four dark stripes on the neck. which are replaced by a series of dark blotches or bands on the posterior portion of the snake's body. They are striped anteriorly, and banded or blotched posteriorly. The dorsal ground color of this species is gray to olive, and the anterior stripes may be darker than the ground color. It exhibits reddish belly scutes, with each bearing a yellowish midventral spot.[7] The diet of this species is the same as the Gulf salt marsh snake, consisting of fish and crustaceans. As with the Gulf salt marsh snake, this subspecies is diurnal during cool weather, and nocturnal during the hot summer nights.[7]
Taxonomy
Some sources consider the three races of N. clarkii to be subspecies of the southern water snake, Nerodia fasciata.[14][15] Others consider not only the three races of N. clarkii, but also the species N. fasciata itself, all to be subspecies of N. sipedon.[16][17]
Subspecies
The following three subspecies of N. clarkii are recognized as being valid, including the nominotypical subspecies.[3]
- Nerodia clarkii clarkii (Baird & Girard, 1853) – Gulf salt marsh snake
- Nerodia clarkii compressicauda (Kennicott, 1860) – mangrove salt marsh snake
- Nerodia clarkii taeniata (Cope, 1895) – Atlantic salt marsh snake
References
- ↑ Hammerson, G.A. (2007). "Nerodia clarkii". IUCN Red List of Threatened Species. 2007: e.T63852A12722122. doi:10.2305/IUCN.UK.2007.RLTS.T63852A12722122.en. Archived from the original on 24 December 2017. Retrieved 23 December 2017.
- 1 2 "Nerodia clarkii". NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer. Arlington, Virginia: NatureServe. 7 April 2023. Archived from the original on 8 April 2023. Retrieved 7 April 2023.
- 1 2 "Nerodia clarkii (BAIRD & GIRARD, 1853)". reptile-database.org.
- ↑ "Saltmarsh Watersnake". ufwildlife.ifas.ufl.edu. Retrieved 4 June 2023.
- ↑ Beolens, Bo; Watkins, Michael; Grayson, Michael (2011). Nerodia fasciata clarkii. Baltimore: Johns Hopkins University Press. p. 55. ISBN 978-1-4214-0135-5.
{{cite book}}:|work=ignored (help) - ↑ "Saltmarsh Snake". Florida Snake ID Guide. Archived from the original on 20 March 2023. Retrieved 2 June 2023.
- 1 2 3 4 5 6 7 8 Bartlett, Richard D.; Bartlett, Patricia (16 December 2003). Florida's Snakes: A Guide to Their Identification and Habits. University Press of Florida. pp. 81–83. ISBN 978-0-8130-2636-7.
- 1 2 3 4 5 6 7 Tennant, Alan (28 February 1997). A Field Guide to Snakes of Florida (1st ed.). Gulf Publishing. pp. 151–153. ISBN 978-0-87719-291-6.
- ↑ "Gulf Salt Marsh Snake (Nerodia clarkii)". tpwd.texas.gov. Archived from the original on 16 April 2023. Retrieved 2 June 2023.
- ↑ Pettit, Rebekah (9 August 2018). "Mangrove Saltmarsh Snake Herpetofauna Field Methods". Reptiles Magazine. Archived from the original on 9 February 2023. Retrieved 2 June 2023.
- 1 2 "Atlantic salt marsh snake". Florida Fish And Wildlife Conservation Commission. Archived from the original on 21 May 2023. Retrieved 2 June 2023.
- ↑ "Atlantic salt marsh snake (Nerodia clarkii taeniata)". Environmental Conservation Online System. U.S. Fish & Wildlife Service. Archived from the original on 8 April 2023. Retrieved 7 April 2023.
- ↑ 42 FR 60743
- ↑ Conant R (1975). Natrix fasciata clarki (Second ed.). Boston: Houghton Mifflin. pp. 147–148. ISBN 0-395-19979-4.
Plates 1-48 N. f. taeniata, p. 148; N. f. compressicauda, pp. 148-149; Plate 21; Map 101)
{{cite book}}:|work=ignored (help) - ↑ Brodie ED Jr (1982). Nerodia fasciata clarki, N. f. compressicauda, N. f. taeniata. New York: Golden Press. pp. 156–157. ISBN 0-307-13666-3.
Plates 1-48
{{cite book}}:|work=ignored (help) - ↑ Schmidt KP; Davis DD (1941). Natrix sipedon clarkii, N. s. compressicauda. New York: G.P. Putnam's Sons. pp. 222–224.
Figure 72 [map] on p. 221
{{cite book}}:|work=ignored (help) - ↑ Wright AH; Wright AA (1957). Natrix sipedon clarki, N. s. compressicauda. Ithaca and London: Comstock. pp. 515–522.
1,105 pp. (in 2 volumes) , Figures 151–154; N. f. taeniata, pp. 541–544, Figure 161; Map 40 on p. 491
{{cite book}}:|work=ignored (help)
Further reading
- Baird, S.F.; Girard, C.F. (1853). Part I.—Serpents. Washington, District of Columbia: Smithsonian Institution.
Regina clarkii, new species, p. 48
{{cite book}}:|work=ignored (help) - Boulenger, G.A. (1893). Containing the Families ... Colubridæ Aglyphæ, part. Vol. I. Taylor and Francis, printers. London: Trustees of the British Museum (Natural History).
Plates I-XXVIII. (Tropidonotus clarkii, p. 238)
{{cite book}}:|work=ignored (help) - Conant, Roger; Bridges, William (1939). What Snake Is That?: A Field Guide to the Snakes of the United States East of the Rocky Mountains. New York and London: D. Appleton-Century Company.
(With 108 drawings by Edmond Malnate); Frontispiece map + viii + 163 pp. + Plates A-c, 1–32. (Natrix sipedon clarkii, pp. 105–106 + Plate 20, Figure 57)
- Cope, E.D. (1895). "On some new North American Snakes". American Naturalist. 29: 676–677.
Natrix compressicauda tæniata, new subspecies
- Kennicott, Robert (1860). "Descriptions of New Species of North American Serpents in the Museum of the Smithsonian Institution". Proceedings of the Academy of Natural Sciences of Philadelphia. Washington. 12: 328–338.
Nerodia compressicauda, new species, pp. 335–336
- Powell, Robert; Conant, R.; Collins, J.T. (2016). Nerodia clarkii. Boston and New York: Houghton Mifflin Harcourt. pp. 414–415. ISBN 978-0-544-12997-9.
Plate 40
{{cite book}}:|work=ignored (help)

