 | |
2.JPG.webp) | |
| Names | |
|---|---|
| IUPAC name
nickel;N-[(Z)-3-nitrosobut-2-en-2-yl]hydroxylamine | |
| Other names
Bis(butanedione dioximato)nickel | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C8H14N4NiO4 | |
| Molar mass | 288.917 g·mol−1 |
| Appearance | red solid |
| Density | 1.698 g/cm3 |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H315, H317, H319, H335, H351 | |
| P201, P202, P261, P264, P271, P272, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
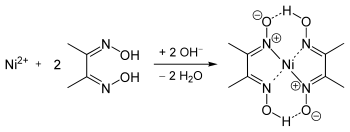
Nickel bis(dimethylglyoximate) is the coordination complex with the formula Ni[ONC(CH3)C(CH3)NOH]2. The compound is a bright red solid. It achieved prominence for its use in the qualitative analysis of nickel.[1]
Structure
The geometry of the nickel(II) ion is square planar.[2] It is surrounded by two equivalents of the conjugate base (dmgH−) of dimethylglyoxime (dmgH2). The pair of organic ligands are joined through hydrogen bonds to give a macrocyclic ligand. The complex is distinctively colored and insoluble leading to its use as a chelating agent in the gravimetric analysis of nickel.
The use of dimethylglyoxime as a reagent to detect nickel was reported by L. A. Chugaev in 1905.[3]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Donald E. Williams; Gabriele Wohlauer; R. E. Rundle (1959). "Crystal Structures of Nickel and Palladium Dimethylglyoximes". J. Am. Chem. Soc. 81 (3): 755–756. doi:10.1021/ja01512a066.
- ↑ Tschugaeff, Lev (1905). "Über ein neues, empfindliches Reagens auf Nickel" [About a new, sensitive reagent on nickel]. Berichte der Deutschen Chemischen Gesellschaft (in German). 38 (3): 2520–2522. doi:10.1002/cber.19050380317.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.