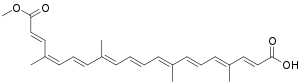
 | |
| Names | |
|---|---|
| IUPAC name
(2E,4E,6E,8E,10E,12E,14E,16Z,18E)-20-Methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18-nonaenoic acid | |
| Other names
cis-Bixin; α-Bixin; 9-cis-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.499 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C25H30O4 | |
| Molar mass | 394.511 g·mol−1 |
| Appearance | Orange crystals |
| Melting point | 198 °C (cis-isomer) 217 °C (trans-isomer) |
| Insoluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bixin is an apocarotenoid found in the seeds of the achiote tree (Bixa orellana)[2] from which it derives its name. It is commonly extracted from the seeds to form annatto, a natural food coloring, containing about 5% pigments of which 70–80% are bixin.[3]
Applications
 Red seeds of the achiote tree
Red seeds of the achiote tree Bixin is one of the colorants used in the snack Cheetos.
Bixin is one of the colorants used in the snack Cheetos.
Several thousand tons are harvested annually.[4]
Chemical properties
Bixin is unstable. It isomerizes into trans-bixin (β-bixin), the double-bond isomer.[1]
 Chemical structure of trans-bixin
Chemical structure of trans-bixin
Bixin is soluble in fats and alcohols but insoluble in water. Upon exposure to alkali, the methyl ester is hydrolyzed to produce the dicarboxylic acid norbixin, a water-soluble derivative.
 Chemical structure of norbixin
Chemical structure of norbixin
References
- 1 2 Merck Index, 11th Edition, 1320
- ↑ Bouvier, Florence; Dogbo, Odette; Camara, Bilal (2003). "Biosynthesis of the Food and Cosmetic Plant Pigment Bixin (Annatto)". Science. 300 (5628): 2089–2091. Bibcode:2003Sci...300.2089B. doi:10.1126/science.1085162. ISSN 0036-8075. JSTOR 3834418. PMID 12829782. S2CID 560600.
- ↑ Executive Summary Bixin Archived July 21, 2011, at the Wayback Machine, National Toxicology Program
- ↑ Stringheta, Paulo C.; Silva, Pollyanna I.; Costa, André G.V. (2018). "Annatto/Urucum— Bixa orellana". Exotic Fruits. pp. 23–30. doi:10.1016/B978-0-12-803138-4.00006-X. ISBN 9780128031384.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
