 | |
| Clinical data | |
|---|---|
| Other names | F901318 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
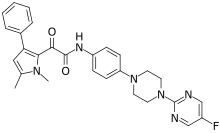
| Formula | C28H27FN6O2 |
| Molar mass | 498.562 g·mol−1 |
Olorofim (F901318) is an experimental antifungal drug being developed for invasive mold infections.[1][2] If approved it would be a first-in-class medication (for the orotomide class). In 2023, the FDA decided not to approve the drug and to request more data about the safety of the drug.[3] Olorofim does not inhibit growth of Candida species.[2]
References
- ↑ Neoh, Chin Fen; Jeong, Wirawan; Kong, David CM; Slavin, Monica A (2023). "The antifungal pipeline for invasive fungal diseases: what does the future hold?". Expert Review of Anti-infective Therapy. 21 (6): 577–594. doi:10.1080/14787210.2023.2203383.
- 1 2 Wiederhold, Nathan P. (2020). "Review of the Novel Investigational Antifungal Olorofim". Journal of Fungi. 6 (3): 122. doi:10.3390/jof6030122. ISSN 2309-608X. PMC 7557671. PMID 32751765.
- ↑ "FDA Passes on Olorofim Despite Critical Need for Antifungals". Medscape.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.